We understand in amazing detail how a heart develops - in mice. Whether the same processes that produce mouse heart tissue also generate heart tissue in humans has been unclear, because we obviously can't do the required experiments on human embryos. But a paper published on Thursday in Nature describes research that used human embryonic stem cells to generate human heart cells, and in the process demonstrated that human and mouse stem cells use similar molecular signaling pathways to develop, or differentiate, from stem cells to various types of heart cells. What this means is that we now have the molecular recipe needed to grow heart tissue from embryonic stem cells. Having that recipe in hand brings us a step closer to an embryonic stem cell-based treatment for damaged hearts.
Embryonic Stem Cell Decision Making
Embryonic stem cells are remarkable because unlike differentiated cells, they have the ability to keep dividing without limit, perpetuating themselves indefinitely. But for medical treatments, they are largely useless in this form: we really want to use stem cells to produce other useful types of cells, like neurons or cardiomyocytes, to replace damaged spinal cords or hearts. Stem cells can become many different types of cells, so the trick is coaxing them to become the type of cell you need. This is not necessarily easy, because you don't go straight from a stem cell to a fully mature, differentiated cell. Stem cells start out pluripotent, able to become many different types of cells, but they then, step by step, commit to an ever more narrow range of possibilities.
Here is a very simplified example of how a stem cell becomes an oligodendrocyte: an embryonic stem cell makes a decision to become part of the ectoderm germ layer, foreclosing its options of becoming a member of the mesoderm or endoderm; in due time that stem cell limits its potential to some type of neuronal cell, then chooses to restrict itself to transforming into glial cell, and finally it becomes limited to oligodendrocytes. I've left out many intermediate steps, but the idea is clear: a stem cell has to follow a path involving multiple decision points to reach its final destination; you can't simply go from an embryonic stem cell to a fully differentiated heart, nerve or muscle cell in one step.
The ability to coax stem cells down the right path of any decision tree is the holy grail of stem cell research. But how can you convince a stem cell to make the right decision? The key is to treat cells with the right combination of signaling molecules, small proteins or other biological molecules that flip a set of genetic switches inside the cell. Each stem cell decision happens primarily because the cell is induced to switch some genes on and others off. Getting the right combination of genes on and off is controlled by these signaling molecules or proteins, which usually have colorful names often inspired by genetic experiments in non-mammals, names like dickkopf (check your German dictionary) and sonic hedgehog.
Stem Cells to Heart Cells
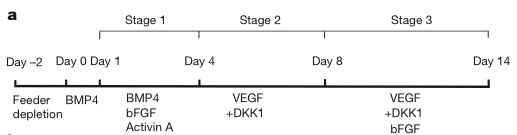
In this case, what the stem cell research group (from Mt. Sinai School of Medicine) did was use the right combination of signaling molecules at the right time to drive stem cells to become various heart tissue cells. The researchers knew that in mice, differentiated heart cells descend from a type of precursor stem cell called Flk-1+ (named for a specific type of protein present in these cells). That is, in mice, the stem cell decision tree to make heart cells goes like this: embryonic stem cells commit to being mesoderm cells, and then, a few decision points later, become Flk-1+ stem cells: stem cells that can form three types of heart tissue, the cardiac muscle, the endothelium, and the vascular smooth muscle. So the researchers were facing two questions: Are there analogous Flk-1+ stem cells in humans, and can we manipulate human embryonic stem cells in a petri dish to become these Flk-1+ cells?
The answer to both questions is yes. The scientists were able to produce human Flk-1+ cells (which aren't actually called Flk-1+ cells, but I'm going to ignore that to avoid confusion) by following a molecular recipe, which called for various signaling molecules at the right time (the abbreviations stand for the various signaling proteins added to the petri dish - DKK1 = dickkopf1, BMP4 = bone morphogenic protein 4, etc):
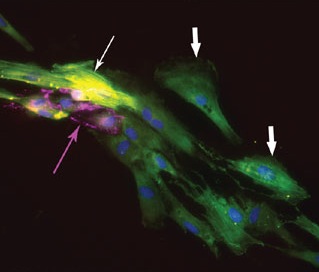
Once they had Flk-1+-like stem cells, the researchers were able to prompt those stem cells to differentiate into various specialized heart cells, including actual beating heart cells. To follow the changes in gene expression during this process, and to ensure that they were seeing the right cell types, the researchers looked for key marker proteins that should be present only at certain points along the decision tree: at one point, you should have cells that express the proteins CTNT and SMA, at another point the cells should express SMA but not CTNT, etc. The way to see whether these proteins are present is to stain the proteins so that they fluoresce under the microscope, producing some of the most appealing pictures you'll find in a research paper:

So the researchers could make these cells in a dish, but what happens in a live animal? To find out, they transplanted the Flk-1+-like, heart precursor stem cells into mice, to see if these cells would turn into heart muscle, endothelium, and vascular smooth muscle. To distinguish the transplanted human cells from the mouse's native cells, the scientists labeled th human cells with a green protein. And sure enough, they found that the transplanted human stem cells had integrated themselves into the mouse hearts and differentiated into the three specific heart cell types. In other words, scientists have turned human embryonic stem cells into heart stem cells, transplanted them into live mice, and observed those cells become functional members of the mouse's heart. The next big step is to use this same technique to improve human hearts.
This kind of tissue engineering in a dish obviously holds great medical promise. Scientists hope to be able to take embryonic stem cells and use them to create replacements for any kind of damaged tissue. To do this, we need the molecular recipe for creating each type of tissue, not an easy task, but clearly, as this research demonstrates, within our abilities.




Comments