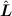 is "quantized" and its z-projection
is "quantized" and its z-projection To see it, let us consider two spinless particles with masses m1 and m2 coupled with any central potential, say, with
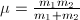 describes the relative particle motion and depends on both particles in a two-particle compound system. It is its orbital momentum which is quantized in terms of integer numbers, namely,
describes the relative particle motion and depends on both particles in a two-particle compound system. It is its orbital momentum which is quantized in terms of integer numbers, namely, The particle-1 and particle-2 orbital (angular) momenta
In particular,
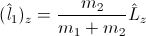 and
and 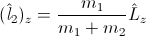 are not obliged to have integer values! Why? Because they are simply non-integer functions of operator
are not obliged to have integer values! Why? Because they are simply non-integer functions of operator Surprise! And the same statement is valid for the positron
The particle orbital momenta in a bound system with respect to the system center of inertia are not momenta of independent, non-correlated subsystems. On the contrary, they are related and the rule of addition of independent angular momenta, usually taught in courses of Quantum Mechanics, is not applicable to them.




Comments