Organic
chemists are regularly surrounded by chemicals and their smell. Colour is not
the only characteristic by which we recognize compounds. Too often, it is their
odor that allows us know that they are around. My relationship with strong,
pungent, fishy, offending odors began in the early years of undergraduate lab
training. This is not true only for chemists, but also for them coming from
allied fields.
Odor is complex chemistry. One can come across various smelly
substances especially those emanating from the kitchen of every household. Odor
Chemistry is so complex that it took until 2004 for a Nobel Prize to be awarded
for work that teased out the nature of smell and the remarkable combinatorial
mechanism by which the human nose senses odor.
Smells
influence much of our behaviour, including what we choose to eat, with whom we
flirt, and also alert us to danger. But, despite its importance, we have never fully
understood how we smell. The olfactory system is a complex set of processes
that include membrane receptors in the nose, electrical signals, and our brain.
However, humans would not be able to detect smells unless the appropriate odor
molecules are released into the air. Scientists from French National Research
Institute for Agricultural Research (INRA) in Jouy-en-Josas, France, have used
lab-on-a-chip technology to shed some light on this complicated process.
Scientists know that aroma molecules, or odorants, bind to olfactory receptors
(ORs) which sit under a layer of mucus in the upper section of the nose. There
are more than 350 different ORs in humans, and these work in a combinatorial fashion
to allow us to smell many more odorants. Odorant binding to an OR sets off a
chain of events that converts the chemical binding energy into a neural signal,
which we register as a smell. What is puzzling, though, is how this first
binding step works – most odorants are hydrophobic, while the mucus covering
the ORs in the nose is aqueous. Scientists have assumed that another species
becomes involved to help shuttle the odorant through the mucus layer: an
odorant binding protein (OBP).
The
main quality of a chemical compound that enables us to smell is its
volatility. The compound should have a
relatively low vapor pressure that allows whiffs of it to escape from its
container and interact with the biochemical sensory switches inside our body. A
lot of organic compounds have this quality so most young chemists encounter
some kind of smell during their freshman or sophomore chemistry lab. As a
newbie, it can be confusing to smell certain compounds that we end up confusing
our senses.We have to remember that human nose is not that good in sensing
odour. If we could, I am sure we would find the smell of the armpit odor
seductive.
Recently, while conducting practicals on microscale separation of
organic compounds from a binary mixture made me think about the smell of
organic compounds. One of my students started sniffing in full curiosity to
identify the separated compound with just a whiffy smell. It is obvious for him
to instinctively start sniffing without realizing the toxic reactions. I
reached his table to make him and his colleagues aware of the harmful effects
of smelly-nonsmelly organic compounds. It also led me to an idea to put up a chart on
the same matter and will be completing it soon.
During
the early days of chemistry, when there were no techniques for determining the
structure and identities of molecules, colour and smell were the two main
qualities on which chemists could rely on for identifying specific compounds.
Even forensic investigators often identified the presence of poisons by their
smells. For instance arsenic has a garlic-like smell, and hydrogen cyanide
smells mildly of bitter almonds. And yes, I so love the smell of bitter
almonds! Unfortunately not all poisons have a compelling odor. Carbon monoxide
is a notorious example, and a lot of deaths from the gas occur because people
cannot smell it while it’s building up around them. Sarin gas is another
example from the lot. Researchers came up with idea of spiking the noxious gas
with smelly compounds, like natural gas, a potentially dangerous odorless agent
can be spiked with minute concentrations of a highly smelly additive methane
thiol.
This brings us to thiols, bad smelly skunks. If you ask chemists to
universally agree upon one element in the periodic table under the title of
king of bad smells, they will affirmatively settle on sulphur, especially in
the form of thiols. Thiols – also called mercaptans – are compounds with a
sulfur bonded to a hydrogen, an atomic combination denoted by SH. Yes, the very
own skunky smell and flatulence. These compounds, along with related thioethers
like dimethylsulfide, are also characterized by their extremely noxious odors
(reminiscent of rotting eggs, overcooked cabbage, sweat, diesel fumes and a
host of other foul aromas) at anything above the lowest of concentrations.
I
was always intrigued by the Kipp’s apparatus generating the simplest thiol-
hydrogen sulfide, denoted by H2S. H2S contributes to the
classic smell of rotten eggs-Some Kitchen Chemistry here. The apparatus
contained a few filings of iron sulfide in hydrochloric acid. The reaction
between these two generated the gas which we would bubble into test-tubes for
semi-micro inorganic analysis.
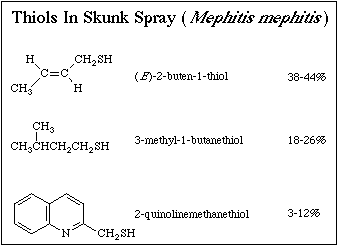
On a quick literature survey, I came across some great snippets on Odor Chemistry. The worst smell ever recorded that led to evacuation of German city, Frieberg (1889) when workers at the Esso Research Station in England were trying to make thioacetone from trithioacetone.

They described the event as,
“……... we found ourselves with an odour problem beyond our worst expectations. … Two of our chemists had done no more than investigate cracking of minute amounts of trithioacetone found themselves the object of hostile stares in a restaurant and suffered the humiliation of having a waitress spray the area around them with a deodorant………..The odours defied the expected effects of dilution since workers in the laboratory did not find the odours intolerable … and genuinely denied responsibility since they were working in closed systems. To convince them otherwise, they were dispersed with other observers around the laboratory, at distances up to a quarter of a mile, and one drop of either acetone gem-dithiol or the mother liquors from crude trithioacetone crystallisations were placed on a watch glass in a fume cupboard. The odour was detected downwind in seconds………..” There are two candidates for this dreadful smell- propane dithiol and 4-methyl-4-sulphanylpentan-2-one.
To work up such a reaction will be a daunting task for a chemist.
"The offensive odors released by cracking trithioacetone to prepare linear poly(thioacetone) are confined and eliminated by working in a large glove box with an alkaline permanganate seal, decontaminating all apparatus with alkaline permanganate, eliminating obnoxious vapors with nitrous fumes generated by a few grams of Cu in HNO3, and destroying all residues by running them into the center of a wood fire in a brazier."
To redeem the honour of sulfur compounds, I must cite the example of truffle which pigs can smell through a metre of soil and whose taste and smell is so delightful that truffles cost more than their weight in gold. Damascenones are responsible for the smell of roses.
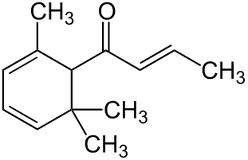
If you smell one drop you will be disappointed, as it smells rather like turpentine or camphor, but next morning you and the clothes you were wearing will smell powerfully of roses. Just like the compounds from trithioacetone, this smell develops on dilution.
Everyone relishes garlic bread but even one bite later you realize that not just your mouth but even your sweat smells like garlic. Garlic is made up of sulphuric compoundsthat render the pungent smell to it. Also, when we put garlic in our mouth, it encourages the growth of certain bacteria that is already present in our mouth. This leads to bad breath. Garlic contains allyl methyl sulphide, which is the reason for the pungent smell. It passes into our blood stream during the digestion process. Once it is in our body, it gets to the pores of our skin and when we sweat, it gets expelled and causes the sweat to smell. The allyl methyl sulphide also enters our lungs and contaminates the air inside. As we breathe, the air enters our lungs, gets contaminated and comes out as we exhale. This is why our breath smells. The effect of this chemical lasts for few hours but the bad breath and body odour will continue till it is completely thrown out of our system by way of sweat or excreta.
If you’ve smelled a durian even once, you probably remember it. Durians have a notorious aroma likened to rotting meat, turpentine and gym socks. Even with the husk intact, the notorious Asian fruit has such a potent stench that it’s banned on the Singapore Rapid Mass Transit.

Food writer Richard Sterling has written “its odor is best described as…turpentine and onions, garnished with a gym sock. It can be smelled from yards away.” The fruit’s flesh is sometimes eaten raw, or is cooked and used to flavor a number of traditional Southeast Asian dishes and candies. It’s also used in traditional Asian medicine, as both an anti-fever treatment and a aphrodisiac. In breaking down aroma extract, taken from Thai durians, with a mass spectrometer and gas chromatograph, the team, led by Jia-Ziao Li, pinpointed 50 discrete compounds in the fruit responsible for its uncommon aroma. Those compounds included eight that hadn’t been detected in durians before—and four compounds that had been completely unknown to science.Their analysis suggests that it is not any single compound but instead the mixture of different chemicals that produces the fruit’s powerful stench. The compounds are identified by their chemical formulae, which are likely cryptic to anyone without a degree in organic chemistry (1-{[1-(ethylsulfanyl)ethyl]sulfanyl}ethanethiol, for example), but the research team associated each one with a particular odor. What’s interesting is that none of the compounds individually seem to match with the characteristic durian smell—they range widely, and include labels like fruity, skunky, metallic, rubbery, burnt, roasted onion, garlic, cheese, onion and honey. A number of them have been detected in just a few other substances, such as cooked beef, yeast extract, dried squid and leeks. Somehow, the combination of these 50 chemicals produces the powerful scent that has entranced and repulsed people the world over.
However, there are innumerable examples of chemical reactions giving pleasant odors too. Maillard reaction is a form of non-enzymatic browning- a chemical reaction between an amino acid and a reducing sugar, usually requiring heat. Reactive carbonyl group of sugar reacts with nucleophilic amino group of the amino acid, and forms a complex mixture of poorly characterized molecules responsible for a range of odors and flavors. This process is accelerated in an alkaline environment, as the amino groups are deprotonated resulting in increased nucleophilicity. The type of the amino acid determines the resulting flavor. This reaction is the basis of the flavoring industry. That is how we can enjoy bread toast, biscuits, French fries and our roasted meat !!!
Addendum: In response to the fiasco to convert trithioacetone to thioacetone.
Organic chemists have experienced lab odors and thus have been exposed to low concentrations of a variety of toxic compounds. Most of the chemists I know are both healthy and long-lived, especially with the one I got an opportunity to work with came from BARC Radiation Unit still rocking at 83. Is there anything to the theory that extremely low concentration chemical exposures help our immune systems? Does the old saying, 'The one that does not kill us makes us stronger” apply here?





Comments