My plan was to figure out which techniques of healthy eating would improve weight and blood sugar in an "at risk" pilot group and if successful, implement the program for the whole company in the following years. Knowing that obesity was both difficult to treat and slow to manifest measurable outcomes in terms of health cost, I proposed that we focus on lifestyle issues of those at highest risk of metabolic problems: workers whose blood sugar had already risen to a high borderline level. If we could arrest or reverse the signs of impending diabetes in this group, it would likely show obvious early health benefits and cost savings for the company. This would come through fewer doctor's visits, prescription medications. . . perhaps we'd even avoid some heart attacks. We started with the borderline glucose group, then sent invitations to anyone in the company whose wellness panel showed two more additional risk factors: obesity, low HDL cholesterol, high triglycerides, or high blood pressure. Twenty individuals signed up and we soon began our pilot with the help of a dietitian and athletic trainer.
My surprise came on the first day of clinic: None of the clients were very heavy. We counseled ten people on diabetes risk, exercise and diet who didn't appear any heavier than the professionals counseling them. The second day of clinic came and went. . . and still, only a couple individuals had a clinically meaningful weight issue to discuss. Confused, I reviewed the data and our methods of choosing our candidates and realized something important in the database of the entire screened population: weight and blood sugar didn't correlate very well. I ran calculations in excel (and I've repeated this procedure in other, larger groups) and there simply was no relationship. This ran contrary to how I had learned about these illnesses, but I soon realized what was going on: by focussing on glucose in a young healthy working group, we were pulling out the most metabolically susceptible individuals, who were not necessarily those that had become obese. . . yet.
I realized that the correlation between obesity and diabetes that is often reported in large cross-sectional surveys doesn't tell us anything about the sequence of events, or about cause and effect. I had always assumed that people first over-eat, then become obese, that too much fat tissue made them insulin resistant and if they went on getting heavier, they would eventually meet full criteria for diabetes. This group showed me that it doesn't happen in that order. The people I was working with were both "pre-diabetic" and "pre-obese." The two diseases were coming on in parallel, due to some other factor. . . call it "X."
When I was in medical school in the 1990s, we still called it Syndrome X. The syndrome was conceptualized and described by Gerald Reaven, a Stanford endocrinologist doing laboratory research on lipids, who gave a ground breaking lecture at an American Diabetes Association meeting in 1988. In that lecture, he laid out his two decades of incremental accumulation of evidence that insulin resistance was responsible for as many, if not more, heart attacks as high LDL cholesterol. He argued that his lab and many others had shown that high triglycerides in the blood, along with poor tolerance to glucose, low HDL cholesterol and hypertension were all mediated by insulin. These factors, he argued, caused the 50% of heart attacks that could not be explained by the high cholesterol model and perhaps suggested an alternate way of looking at why coronary artery disease and strokes occur.
Why he chose to name it syndrome "X" as if the cause was unknown, rather than "the insulin resistance syndrome" is something I can't understand. I do know that in 1993, in my basic medical school lectures, when the idea was still working its way into the consciousness of researchers and clinicians, the name made it sound mysterious. We didn't think of insulin as having anything to do with fat handling and we were taught that obesity was a behavioral disorder (one that we didn't spend much time on, as a matter of fact). The Syndrome X nomenclature seemed to hide the point that insulin resistance was the missing factor doctors were not seeing.
A decade later, as I puzzled over what to do with my "pre-obese" clients who were showing signs of all the metabolic abnormalities described by Reaven, the cluster of findings had since been re-named to metabolic syndrome. But that still didn't help me know what to do next. Was there a diet and exercise program that directly addressed the cause of metabolic syndrome? Calling it "insulin resistance syndrome," wouldn't have made it any easier for me to proceed. The question was: why were people becoming insulin resistant in the first place? Insulin resistance wasn't an ultimate cause, but a marker and an expression of something else. I got to work reading the papers published by Reaven and others.
As the idea of "low carb" was beginning another trendy phase at that time, I was surprised to see Dr. Reaven had been cautioning against low fat diets since the mid-sixties, contending that they were unlikely to help LDL and very likely to cause worsening triglycerides and blood sugar. He had warned in the early 1980s that universal adoption of low fat diets were misplaced and bound to harm those for whom LDL was never a problem. He seemed like a prophet when I read him in 2003 (His 2000 book committed egregious acts of disease mongering, but was full of good science too), but more importantly, his papers and those of researchers who followed his reasoning, showed me what was happening in my clients' bodies. The following is my summary of his teaching.
There are three different metabolic scenarios: one type of person is normal, with blood pressure under control, LDL and HDL in the normal range, triglycerides normal, and blood sugar normal. This is the most common finding of any group you would usually screen at a yearly company wellness drive. The second scenario is the one your doctor is most familiar with: most of the numbers are normal, but your LDL, or "bad" cholesterol is too high. This is easily remedied by taking lipitor or any of the other "statin" drugs, which most doctors are quick to do. Treating LDL is one of the most common general medicine activities around and it's straight out of the traditional view that high cholesterol causes build up of fat in the coronary arteries that eventually become big enough to stop blood flow and cause a heart attack. The third metabolic profile is actually pretty strange: blood pressure is up, blood sugar is borderline high, triglycerides are high, LDL is normal and HDL, or "good" cholesterol is low. The LDL is actually "small and dense" in character, making it more likely to cause problems, but the number is normal on the lab test. This is the metabolic syndrome. Dr. Reaven showed that all of these happen in the context of insulin resistance with the most direct cause being a high carbohydrate diet. To understand why, we need to think about cholesterol in a little more detail.
LDL and all the rest are “lipoproteins.” They are measurable globs of fatty stuff in our blood that can be separated and counted. The globs are of different sizes and densities. There are actually hundreds of different-sized globs of fat circulating in the blood, but we focus on the few for which we have a decent guess regarding their purpose in the body. The HDLs are “high density” and if you were to eyeball a test tube of human plasma, they’d be down near the bottom, because they are densest. The LDLs would rise to the top like cream, because they are “low density.” What makes them light or dense, in the simplest sense, is, whether they are they full of fat or not. They also have different roles in moving lipids about. LDLs, in general, are heading out into the circulation, while HDLs are heading back to the liver. HDLs represent a particle that has capacity to pick up cholesterol molecules. If you eat too much, or make too much, cholesterol, HDL can take it back to the liver and out of circulation before it deposits in your artery wall. LDLs are basically full of fat and cholesterol and they are about to disgorge it somewhere you probably do not want it.
Cholesterol science is confusing and the terminology doesn’t do much to help. For starters, we call HDL and LDL “cholesterol,” which isn’t accurate. They transport cholesterol. Also, we call them “lipoproteins” but they really don’t perform like proteins and aren’t composed of amino acid chains like a protein. They are lipid droplets. They have proteins in their outer walls that help them get absorbed into different tissues, but they really aren’t proteins in any real sense. They are, as I say, best thought of as a bunch of globs floating around, carrying cholesterol and triglycerides.
We report a “triglyceride” number in the lab report along with HDL and LDL, but triglycerides are not actually a glob, like LDL or HDL, they are molecules, more like cholesterol itself, which isn't measured directly. They are something that is carried inside the lipoproteins, mostly VLDL (very low density lipoprotein) and chylomicrons which come from the gut after we eat. LDL and HDL also carry some triglycerides, but mostly traffic in cholesterol.
So, what’s up with the lipid profile of the patient with metabolic syndrome? Why don’t they just have a high LDL like regular cardiac patients? What makes this a “syndrome?” The answer comes from the susceptibility to the modern diet. Like most of us, those with metabolic syndrome are eating a high calorie, high fat, high carbohydrate diet (trust me, I review the diet logs). The carbohydrates we eat each meal break down to sugar, which goes to the liver. The liver says, in essence, “good, I’ve got enough sugar for energy, I can get rid of these triglycerides I’ve been storing all day." So high triglycerides (fat in the blood) can be caused by carbohydrate in the diet.
So after a high carb meal, the liver releases VLDL particles, full of triglycerides into the blood. Meanwhile, the fat in the diet (which is primarily triglyceride nearly identical to that made in the liver) is absorbed in the intestines and packaged into particles called chylomicrons. So, after eating a fat and carbohydrate rich meal, we have two reasons for high triglycerides: what we’ve absorbed directly from our meal and what’s been put out from the liver.
Here’s where it gets interesting: HDL and LDL, floating around in the blood stream, are able to exchange some of their contents (cholesterol molecules) with each other and also with chylomicrons and VLDL. This happens in low grade fashion all the time, its just one of the numerous balancing acts the body uses to keep things regulated. However, this ability to exchange lipids between particles can be altered too much in one direction by our dietary habits. When there is a high number of VLDL from the liver (from eating too much carbohydrate) and a lot of chylomicrons (from eating too much fat), more exchange happens than would normally be expected. HDL and LDL give up some of their cholesterol in exchange for triglycerides, so they become “small and dense.” The small HDLs go to the kidney, which takes them out of circulation. This makes our HDL number “low” when we measure it. The LDLs stay in the blood stream as “small and dense,” but their overall number is normal. The triglycerides are measured as high because the high fat, high sugar, high calorie diet makes it high by the method explained above.
This gives us the signature lipid panel of the metabolic syndrome patient: high triglycerides, low HDL and normal (smaller, denser) LDL.
To consolidate the above: the particles are not unique, separate entities, but run a gamut of sizes from high density to very low, with all sizes in between as they morph and bump into each other, exchanging their contents via enzymes like cholesteryl ester transfer protein. Rather than separate entities, it would be simplest to think of them as different versions of the same protein (or “blob” of fat, really) that can present a differing lipid panel on a lab, depending upon what we eat. The high TG, Low HDL, normal LDL profile is simply the human adaptation to a high fat, high carbohydrate diet. Notice that high triglycerides and borderline glucose are what you’d see if you did a non-fasting panel on nearly anyone. These things are supposed to go up if we’ve just eaten. What’s different about our patients with the metabolic syndrome, is that the blood looks like it’s in a “fed” state, even when they’ve fasted overnight. By and large, my obesity patients are not breakfast eaters. Why? Because there’s fuel sitting in the bloodstream already, in the form of a high fasting glucose and triglyceride, so their hypothalamus thinks they’ve been fed. Until the boom and bust of sugar feeding starts later in the day, my patients are relatively hunger free.
What I learned by focusing on my pre-diabetic, "pre-obese" population is that the constant refrain of physicians to their patients, "If you lost a little weight, all of these problems would improve," is actually inaccurate. It assumes that it is weight loss, itself, that causes improvement in the metabolic parameters. In fact, the weight, or more specifically, the increased adiposity, is a symptom, just as lower HDL and higher triglycerides are symptoms. Obesity improves as the other metabolic parameters improve and by the same method: improvement of poor nutrition habits. In the case of the metabolic syndrome clients, this means lowering fat and carbohydrate in the diet.
Getting back to the pre-diabetic workers who signed up to try diet and exercise for diabetes prevention: Our results matched those of many trials in that those that lost weight, also improved blood glucose and the cholesterol ratio. The two moved in parallel in the reverse direction through diet and exercise. As the diabetes prevention program outcomes showed, very little weight loss is needed to improve metabolic syndrome. The human body is not waiting for a reduction in the fat stores before allowing the other markers of metabolic syndrome (the daily blood sugar, triglycerides, the changes in the cholesterol particles) to improve. Food determines the markers, in parallel, or even regardless of weight loss, because reducing the amount of fat on the body doesn't have anything to do with metabolic consequences. The fat itself, the adipose tissue on the body is not the cause of metabolic syndrome, rather, an adaptation to it.
Roger Unger and Phillip Scherer are University of Texas researchers who argue that fat doesn't cause diabetes, but rather helps delay it. The role of the fat tissue, they explain, is to act as a a cushion for excess energy we consume, not only so that we can use it later, but because it can be damaging to the other tissues of the body. While there are a host of behavioral explanations for why dieting doesn’t work, these researchers argue that the most important factor limiting our ability to lose weight is that our body doesn’t share our disdain for the adipose tissue. In fact, the body, the part we like, the muscles and other organs comprising the lean tissue, is quite dependent on fat’s presence. Why? Drs Scherer and Unger believe (and have shown quite clearly in mice) that the fat cells act like an organ whose main purpose is to protect us against the ill effects of overeating. In a 2003 article published in the journal Endocrinology (provocatively titled "Weapons of Lean Body Mass Destruction: The Role of Ectopic Lipids in the Metabolic Syndrome") Dr. Unger shows how much worse off we would be without fat to protect us from ourselves.
Roger Unger is an MD working in diabetes who argues that, in addition to storing energy for potential famine (the traditional view of adipose) the more important, and much more interesting, role of the fat cell is to absorb energy so that it doesn’t go elsewhere and cause damage - the type of damage that leads to diabetes, fatty liver and heart disease. With the exception of fat tissue, no body organ does very well in the presence of fatty acids. If a body did not have the ability to store fat over time, the fat would infiltrate our muscles, liver, pancreas, heart and wreak havoc. . . which is what it actually does when the adipose tissue becomes poorly regulated. Dr. Unger points out that this is actually what “metabolic syndrome” is: our body having trouble coping with excess calories which results in damage to the cells not specifically equipped to handle it. Why do the other tissues have trouble with fat? They lack some of the specialized enzymes that help package and process it. Any cells which receive more fat than is needed for immediate energy needs winds up allowing the fatty acid to go through alternate metabolic pathways that lead to stress on the inner organelles of the cell. This sends distress signals which sometimes result in the cells dying off.
Cell death in response to stress is one of our body’s ways of dealing with toxins. The body needs to shut down the processes of cells that have gone awry in order to keep healthy functioning. So, when things get too out of balance (as in cancer, or infection) the body is pretty good at just killing off problem cells. This causes trouble in the context of overeating and the pancreas, because the fat seems to get compartmentalized in the beta cells that make insulin. The excess fat derails the normal metabolism, the cells send off distress signals and then the beta cells begin to die off. This is the later stage type 2 diabetes: an inability to secrete enough insulin to process the carbohydrates that we eat. This is a little bit different than our typical explanation for diabetes: first you eat too much, then you get fat, then that fat screws up your metabolism and makes you diabetic. In particular, the role of the fat cell, in the eyes of Unger and Scherer, is re-written from villain to hero. As they explain in an article published in Trends in Endocrinology and Metabolism, 2010, the fat cells valiantly combat the ill effects of over-nutrition by expanding their activity, storing energy to protect the more sensitive pancreas, muscle and heart, which don’t deal well with excess calories. The fat cells grow and multiply as a protective adaptation to how we live. They are not the cause of diabetes, but rather, the one thing protecting us from it.
They refer to the process as “lipotoxicity.” The fat that we eat can act like a toxin in our tissues. The fatty acids and triglycerides that are the product of taking in more energy than can be used or stored are harmful locally due to producing inflammatory signals that both damage cells and cause cell death. The lean, healthy body is one that is able to partition and traffic energy into the fat cells to be stored or to muscles to be used. Individuals susceptible to metabolic disorders like diabetes are not as good at sending the fat to the right places and this helps drive the metabolic illnesses. The hormones leptin and adiponectin, both made in the fat cells, play key roles in this process. Leptin, which gets secreted in greater quantities as the fat cells grow, acts in a number of protective ways including serving as a “stop eating” baseline signal to the brain. Adiponectin has several roles as well. A key feature seems to be turning on the cell’s machinery to effectively process fat, rather than letting it cause damage. The levels and balance of these hormones are likely different in people which yields a varying susceptibility to obesity and diabetes. Through describing the mechanisms of these hormones derived from the fatty tissue, Unger and Scherer show us that fat is necessary and vital to our health.
Recall the status of my metabolically at risk patients: our initial group did quite well with no new cases of diabetes in the first year compared to a similar group of patients who chose not to participate. The numbers were too small to say definitively, but I came away convinced that very modest changes in diet could have dramatic effect on risk. As I moved on to work with more patients with a chief complaint of obesity, it seemed that lower carbohydrate diets seemed to improve patient numbers the quickest. Not carbohydrate elimination, resulting in ketosis, but simple lower carb dieting with a focus on calorie counting to avoid overeating. However, there was a fair bit of variety in the results. not all my patients had good luck with a simple "reasonable carbohydrate" prescription of reducing carbs to about 40% of intake. The diabetic patients, in particular, seemed to have more difficulty with low carb advice with diet and glucose tracking logs that didn't match my simple theory very well. Their sugars were all over the map and I didn't know if it was because they were worse at following a lower carb diet, or whether there was something in the actual metabolism that gave diabetic patients a different reaction. Since eating carbs sustains the blood sugar, if we reduce carbs, we have to reduce blood sugar, don't we?
I decided to run an experiment on myself. I already had a glucose meter at home from when I had tried to figure out why I sometimes hit a wall at the two hour mark while biking (it's not low blood sugar - proved that one to myself). The plan was to test the principles of my weight loss program (eat more protein, eat less carb, don't worry about fat) while measuring my blood sugar regularly. After a week, I would revert to my usual devil-may-care diet and count up the difference a few basic changes could make. The second week would of course look like a diabetic, even though I’m healthy. I pictured being able to show some very clear slides at future obesity talks: Week one, behaving well: sugar is smooth. Week two, eat like an American: blood sugar high and erratic.
If the numbers were consistent enough, I thought I might even be able to start estimating what normal vs. controlled carb eating might mean in terms an expected average, or hemoglobin A1C. Or, if it worked well, I could show this to new patients who were still reluctant to believe: "look, this is a graph of my blood sugar with normal eating, look at the difference after I go low carb..."
So what actually happened?
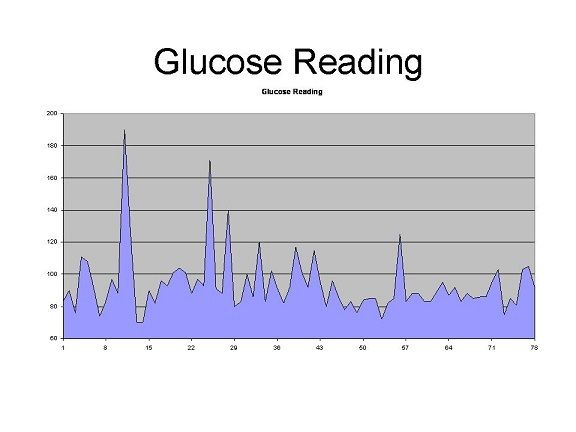
The above shows my blood sugars over what ended up being twelve days of testing. The values up until number 57 are the moderate carb, higher protein diet and the glucose readings after 57 are what happened when I basically went nuts on sugar, eating apple jacks, candy canes, chocolate and drinking coke. It’s easy to see the pattern there. Notice how smooth and low the values are after the 57th reading. My average daily glucose was 95.9 mg/dL on the lower carb diet and 85.6 mg/dL on the higher carb diet. The average increase in glucose after meals the first week was 20.5 mg/dL and only 4.5 mg/dL the second. The controlled diet had much higher spikes in blood sugar than the indulgent, more typically “American” diet. Eating 500-1000 calories more and 100 extra grams of carbohydrate per day, I saw consistently lower blood sugars!
In the second week, I got so frustrated at seeing such a steady stream of blood sugars in the 80s that after a couple days, I began trying with all my might to make my blood glucose go up after meals by ingesting the worst garbage I could think of. One evening I drank three coca-colas in a row, while checking blood sugar every half hour. . . and reached a peak of only 95 mg/dL. A couple days later, I strutted into my coffee shop, determined to show my pancreas who’s boss. I ordered a mocha and a chocolate croissant, then a second mocha and downed the whole 820 calories (with 116 grams of simple sugar) in twenty minutes, thinking, “surely, if ever there was a meal that was created to provoke one’s blood sugar, this is it.” I then checked my glucose every ten minutes to find the spike…which reached 105 mg/dL at 30 minutes before normalizing down to the 80s again. Then I stopped to think about how insulin works.
In a healthy, non-diabetic, non-obese body, insulin is capable of handling doses of incoming calories and sugar without difficulty. Generally within an hour, certainly two, a healthy body will achieve a normal glucose level of less than one hundred, regardless of the meal. Insulin is secreted in two phases. The first phase happens when sweets hit the tongue. The pancreas knows that sugar is coming, so releases a bunch of insulin that is pre-prepared in little packets that are sent into the blood stream. Insulin begins immediately shuttling the glucose out of the blood and into the liver, muscles and fat cells. It then turns on the machinery to make more insulin from scratch, depending upon how much glucose has entered the blood during the meal. That second wave of insulin gets released over thirty to sixty minutes, bringing the blood sugar down to a normal level by hour two, at latest. This is why many of the tests for diabetes check glucose two hours after a known load of sugar. The first phase doesn’t get turned on much by protein or fat and the whole process with low carb meals is a slower reaction which brings the sugar more gradually back down, and not with such a rapid insulin spike. Thus, sugary foods can actually turn on the insulin mechanism better than protein or fat, leaving the blood sugar lower after a high-carb meal than a lower-carb meal. Sugar and insulin are in balance and in tune with each other.
This way of thinking works for a healthy body. Granted, this was a crude experiment, run on one free-living individual, using fingerstick glucose measures, but it is evidence that we need to think twice about giving generic diet advice regarding carbohydrates. A more important question than how one healthy individual's blood sugars look over 12 days is how does this look in those whose insulin is not still doing a good job. How does all this play out in type 2 diabetic patients?
In “A Low Carbohydrate, Ketogenic Diet to Treat Type 2 Diabetes,” researchers Yancy et. al, ran a more professional version of my home-grown experiment. Instead of focusing on the post-meal blood sugars, they waited a full three months to see how the blood glucose would average out in terms of hemoglobin A1C (a measure of how well your blood sugar is controlled over longer periods). They found that a very low carbohydrate diet (under 50 grams per day) lowered the average A1C by 16% even while medications were reduced or stopped in the majority of the volunteers. Their conclusion was that a low carbohydrate diet was more effective than traditional (low fat) diet counseling for diabetics trying to get their glucose down.
One hypothesis for reconciling the conflicting results of my brief experiment and the more formal study above is that I don’t know what I’m doing. Another might be that non-diabetic bodies react to sugar and complex carbohydrates differently than diabetic bodies. What is diabetes but an inability to handle carbohydrate exposure? Just because the main feature of the disease is high blood sugar after ingesting carbohydrates, we cannot assume that carbohydrates cause the disease. That’s as silly as thinking eating cholesterol causes heart disease, just because people with heart disease tend to have high cholesterol in the blood. In both cases, we are mistaking a symptom, or sign, with the cause of the disease. The problem in diabetes is poor blood sugar regulation, not simply ingestion of too much sugar (same goes for coronary artery disease and cholesterol).
In Continuous Glucose Profiles in Healthy Subjects under Everyday Life Conditions and after Different Meals, G. Freckmann and others from a group in Switzerland, used continuous glucose monitors to check the response of healthy adults to different foods. Like me, they used mixed meals containing more or less carbohydrate, protein and fat. But unlike me, they got very accurate blood sugar numbers using the continuous glucose monitors, which track blood sugar every five minutes. Also, unlike me, they found that the blood sugars of the participants responded exactly as biology would predict: higher protein, higher fiber meals had smaller and slower increases in glucose than higher carb, low fiber, low protein meals.
The blood sugar responses in this study seem to be more closely associated with percent of carbohydrates in a meal than the grams. In fact, they kept the amount of carbohydrates in the test meals essentially constant at 50 grams per meal. Fiber seemed to be the best determinant of blood sugar control (my interpretation, looking at their tables) with protein percent and fat percent close behind. The highest fiber, highest protein meals had less than half the increase in blood sugar after a meal, in these healthy subjects, than the lowest fiber, lowest protein meals. Related to my point in my previous post, the ranges were very wide, to the extent that there may have been individuals who reacted exactly opposite to the general trend (some weirdo like me, perhaps). This is why actual studies are so much superior to checking your own glucose in the coffee shop: the average results of a group are much more likely to give logical, true results than individual cases, especially over short periods of investigation.
One surprising feature of the results in this study is that calories (just as I had seen in my body) did not seem to matter. The meal which produced the smallest area under the curve for glucose was 16.5% protein, 26.8% carbohydrate (1/4th of this was fiber), 56.7% fat and 750 calories. The meal with the greatest area under the curve was 14.2% protein, 74.6% carbohydrate (no fiber), 11.2% fat and only 271 calories…Same amount of carbs remember, just mixed to a lesser degree with protein, fat and fiber. Essentially the more dilute and hidden the carbs in the meal (given the same quantity of carbs) the lower the blood sugar. It was not a trivial difference: on the high percent carb meal, blood sugar peaked at 133.2 mg/dL. On the low percent carb meal it reached an average of only 99.2 mg/dL. This is essentially the difference between healthy and unhealthy.
Note that fat is either an innocent bystander to the whole process, or it contributes to lower blood sugar based on these results. They didn’t test that and I don’t want to speculate. But, it must be pointed out that the traditional advice given to diabetics, to eat a low fat diet, has no backing from this small data set.
So, why, considering all this, do dietitians and the endocrinologists who treat diabetes most typically recommend low fat, traditional "heart protective" diets rather than lower carbohydrate? The most obvious answer is that they are not actually "considering all this." A current specialist in diabetes has been trained in a school of thought codified in textbooks which have changed little since the 1990s. If they are paying attention at all, they are probably wondering why their advice fails to achieve good results most of the time. The recent trend has been to do less and less diet teaching and get type 2 diabetic patients on insulin sooner than the past. Many of the most up to date dietitians and nurses certified as diabetes educators have chucked trying to manage diet and have become experts in adjusting insulin instead. Can you blame them? We know diets almost always fail in the end.
Another trend, however, is the increasing recognition that food is the driver of this disease and in particular, that macronutrients matter. There is a slow, steady recognition that low fat diets are a strange treatment for diabetes and it is likely only a matter of time before we see the shift in clinical care toward a higher protein, lower carbohydrate diet.




Comments