

In an elegant study that was reported online first, Maddury Somayazulu and colleagues at CIW obtained pressure-induced bonding and compound formation in xenon–hydrogen solids. What is most remarkable is the stability of the newly discovered and hydrogen-rich Xe(H2)7.
Researchers employed a diamond anvil device to squeeze together a series of gas mixtures of xenon in combination with hydrogen to high pressures. The atoms formed, at about 41,000 times the atmospheric pressure at sea level, a lattice structure rich in hydrogen but interpersed with layers of loosely bonded xenon pairs, or dimers. As the pressure increased to even much higher levels, the distances between the xenon pairs contracted to those observed in dense metallic xenon.
Here is a CIW picture of the newly discovered phenomenon. The red spheres are xenon dimers and the yellow spheres are in essence rotationally disordered (spinning) hydrogen molecules. The picture is supposed to convey two aspects, one, the dimers of xenon dumbells and, two, the packing density that occurs due to high pressures. What the researchers find is that the intermolecular forces change character gradually with increasing pressure while giving rise to novel compounds.
The picture is a structural representation of rhombohedral Xe(H2)7 showing xenon dimers coordinated to rotationally disordered hydrogen molecules and refers to the structure at about 50,000 atmospheres. The dimers exist at that pressure and all the way to 2.5 million atmospheres. The paper addresses at the end what the dimer-dimer distance would be. If that distance were below some critical value, we would have metallic xenon interspacing non-metallic hydrogen. It turns out that while the intra-dimer distance compresses rapidly, the inter-dimer distance does not. That is another mysterious behavior because hydrogen between the dimers is known to be highly compressible.

It is exciting to catch a new finding that might become the next foundation to brand new methods and materials for hydrogen storage.
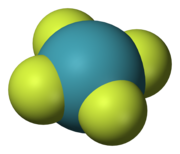
To make a final point, please refer to the structure of xenon tetrachlorde (XeF4). This compound (Xe to F2 mol ratio of 1:2) was the first to be discovered for noble gases like xenon. Now compare it with the structure of Xe(H2)7 (Xe to H2 mol ratio of 1:7) produced by Maddury Somayazulu and colleagues at CIW. It was stated previously in the literature that flouride gas is needed for all xenon compounds. This is not true anymore!
Structure of XeF4 (Credit: Wikipedia)
Notes:
Wendel A. Caldwell, Jeffrey H. Nguyen, Bernd G. Pfrommer, Francesco Mauri, Steven G. Louie, and Raymond Jeanloz. Structure, Bonding, and Geochemistry of Xenon at High Pressures. Science 15 August 1997 277: 930-933 [DOI: 10.1126/science.277.5328.930] (in Reports)
Maddury Somayazulu, Przemyslaw Dera, Alexander F. Goncharov, Stephen A. Gramsch, Peter Liermann, Wenge Yang, Zhenxian Liu, Ho-kwang Mao&Russell J. Hemley. Pressure-induced bonding and compound formation in xenon–hydrogen solids. Nature Chemistry advance online publication Published online: 22 November 2009 doi :10.1038/nchem.445
Image Credit: NERC, NASA, CIW, and Wikipedia, in the presentation order.




Comments