Dear Mickey - How is life? Life is tough, says my friend. Do you agree? I wanted to write to you for a number of reasons. Believe it or not, people still remember that you turned 80 last year: Happy Eighties to you, M-I-C-K-E-Y!
You are shy in public. That is OK because people would prefer a shy mouse any day. Research related to shyness concentrates on humans at this time. Someday someone will report on your kind of shyness, I am certain. You are also a big mouse! Are you up for some mice news? The latest find is hormonal on/off switch for male mice fertility. Michele Welsh and colleagues from UK and Bulgaria found the first evidence to pinpoint how and where androgens work in the testis to control male fertility. Male contraceptives are in the works now with androgens and their cellular receptors. New agents to boost sperm production are also sought for research. You realize, Mickey, the find was with laboratory mice. Two sets of mice, fertile and infertile were involved. Infertility was the result of a missing gene from the peritubular myoid cells in the testis. This gene is the one to code for the androgen receptor. Without the gene, sperm production was reduced to levels too low for fertility.
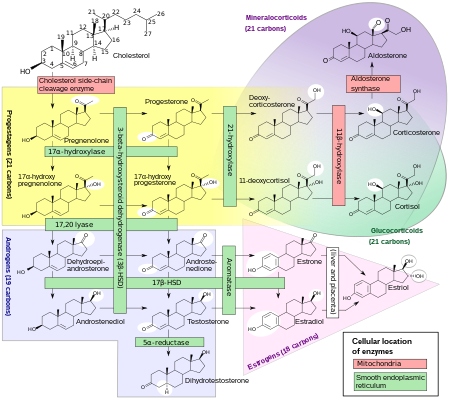
Mickey, here is a lovely chart to explain how all steroid hormones including androgens are made in humans starting from cholesterol. I think future male fertility studies would modify some parts of it. Do you see testosterone (in blue) before it converts to estradiol (in pink), the major estrogen in humans?
Biosynthesis chart of major classes of steroid hormones: progestagens, mineralocorticoids, glucocorticoids, androgens, and estrogens, with some overlap, e.g. mineralocorticoids and glucocorticoids, and white circles for molecular structure changes. (Credit: Wikipedia)
It was Emil Knauer who discovered in 1890 some mysterious substances that control human body processes. These chemicals were named hormones in 1905. Twenty-three years later, coincidentally when you, Mickey, came into being, progesterone (in yellow), the ovarian hormone was identified at the University of Rochester as crucial in pregnancy. A year later, independent of each other, Edward Doisy at Washington University and Adolf Butenandt in Germany isolated the human sex hormone estrogen. Leopold Ruzicka proved that cholesterol could convert into the male hormone androsterone (in blue). When was testosterone identified? To make a long story short, the synthetic testosterone was made independently by both Butenandt and Ruzicka in Europe and earned them the 1939 Nobel Prize in Chemistry.
Mickey, you know androgen (testosterone) affects the whole body of mice or humans, including the brain, bone and muscle, fat distribution, vascular system, genital tissues, and sexuality. Gender blood levels of testosterone vary among individuals and according to their drives. Here is another colorful chart for familiarity. It is a compound image used to interpret blood tests. Adult male testosterone levels are given in light blue on the center's left side. It would have been useful to compare it with a mouse-blood chart. Alas!
Chart of reference ranges for blood tests, adult male testosterone levels in light blue
at center left. (Credit: Wikipedia)
Men and mice are miles apart in fertility, to say it boldly. A gene on/off switch could be real but controlling male fertility is not a piece of cake. A review of the development of female birth control pill uncovers a history of chemicals and female test subjects in the twentieth century. The "female pill" was not developed with laboratory mice. Male mice might not even be the principal model animal for male fertility in mammals, including humans. There could be yet unknown but important differences in the gene(s) responsible for male fertility in mammals. Especially, no offense intended, if the brain is involved in the fertility process in a major way. We already know there are fundamental differences between the brains of humans and mice.
A case in point in recent years is a long-term male contraception with mice. The work targeted the imino sugar N-butyldeoxynojirimycin (NB-DNJ), or miglustat, to cause reversible infertility in male mice. To contrast with the current gene study, this treatment was nonhormonal. The effects on spermatogenesis in mice were found strain-dependent, while in rabbits and men the drug was ineffective.
Do you know about knockout mouse? A knockout mouse is a laboratory mouse whose existing gene has been inactivated, or "knocked out," by either replacing it or disrupting it with an artificial piece of DNA. The figure published by Yildiz Yildiz and colleagues (see below) shows 160 times magnified microscopy of sperm plus egg involving Gba2+/+ mice (left) and Gba2–/– mice (right). Sperms from knockout mice (right) were deficient in zona pellucida binding. By identifying the role in mouse male fertility for GBA2, a protein important in bile acid metabolism, the scientists might find a drug to block the function of GBA2. Will other animals with blocked GBA2 also have impaired fertility? The mouse gene for GBA2 is known to share 83% of its sequence with the human gene. A nonhormonal male contraceptive pill still remains a possibility at this time.
Figure showing failure of sperm from Gba2–/– mice to bind the zona pellucida.*
You have survived wars, b&w TV, and the Beatles. You are a survivor, Mickey. Interestingly, Tomohiro Kono of the Tokyo University of Agriculture found fatherless mice lived 30% longer. The mice had two mothers and were thus female themselves. The female mice were genetically engineered to carry genes from two females. Of course, not limited by genetics, you might live forever and observe further evolution of the biosynthesis chart depicting steroid hormone production from cholesterol.
Notes:
The Pill: pbs.org.
nobelprize.org.






Comments