A new biofuel has been created from yeast and ordinary table sugar. The yeast produces oils and fats, known as lipids, that can be used in place of petroleum-derived products.
It has something everyone can love. And hate. It's a biofuel, which environments love, but the yeast has been genetically optimized, which environments hate. And it uses table sugar, which will make Mark Bittman and New York Times readers recoil in diet fad horror.
But the culture wars aside, the researchers' platform produces the highest concentration of oils and fats reported through fermentation, the process of culturing cells to convert sugar into products such as alcohol, gases or acids. They were able to rewire yeast cells to enable up to 90 percent of the cell mass to become lipids, which can then be used to produce biodiesel.
University of Texas at Austin's Cockrell School of Engineering Assistant professor Hal Alper and a team of students created the new cell-based platform. Given that the yeast cells grow on sugars, Alper calls the biofuel produced by this process "a renewable version of sweet crude."
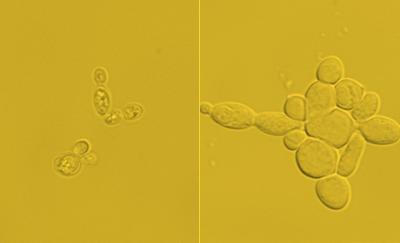
(L) Starting cells with around 15% lipid content. (R) Engineered cells with nearly 90% lipid content. Credit: Cockrell School of Engineering, The University of Texas at Austin
Since fatty materials are building blocks for many household products, this process could be used to produce a variety of items made with petroleum or oils — from nylon to nutrition supplements to fuels. Biofuels and chemicals produced from living organisms represent a promising portion of the renewable energy market. If government subsidies and mandates remain in place, the global biofuels market is expected to double during the next several years, going from $82.7 billion in 2011 to $185.3 billion in 2021.
"To put this in perspective, this lipid value is approaching the concentration seen in many industrial biochemical processes," Alper said. "You can take the lipids formed and theoretically use it to power a car. We took a starting yeast strain of Yarrowia lipolytica, and we've been able to convert it into a factory for oil directly from sugar. This work opens up a new platform for a renewable energy and chemical source."
The biofuel the researchers formulated is similar in composition to biodiesel made from soybean oil. The advantages of using the yeast cells to produce commercial-grade biodiesel are that yeast cells can be grown anywhere, do not compete with land resources and are easier to genetically alter than other sources of biofuel.
So far, high-level production of biofuels and renewable oils has been an elusive goal, but the researchers believe that industry-scale production is possible with their platform.
In a large-scale engineering effort spanning over four years, the researchers genetically modified Yarrowia lipolytica by both removing and overexpressing specific genes that influence lipid production. In addition, the team identified optimum culturing conditions that differ from standard conditions. Traditional methods rely on nitrogen starvation to trick yeast cells into storing fat and materials. Alper's research provides a mechanism for growing lipids without nitrogen starvation. The research has resulted in a technology for which UT Austin has applied for a patent.
"Our cells do not require that starvation," Alper said. "That makes it extremely attractive from an industry production standpoint."
The team increased lipid levels by nearly 60-fold from the starting point.
At 90 percent lipid levels, the platform produces the highest levels of lipid content created so far using a genetically engineered yeast cell. To compare, other yeast-based platforms yield lipid content in the 50 to 80 percent range. However, these alternative platforms do not always produce lipids directly from sugar as the UT Austin technology does.
Alper and his team are continuing to find ways to further enhance the lipid production levels and develop new products using this engineered yeast.
Source: University of Texas at Austin




Comments