A taste of honey, I suggest it is for IBM and the rest of us, to see a molecule. Have you missed the news chockful of opportunities? Before that however, let's remember several people. One chemical engineering graduate, who studied quantum mechanics to develop new rules for the shared electron bond, was Linus Pauling. He mailed in 1931 to the JACS The Nature of the Chemical Bond and received 1954 Nobel Prize in Chemistry for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances.
Top: Linus Pauling lecturing at Caltech in mid 1930s. (Photo credit: Oregon State University)
Bottom: IBM Research - Zurich scientists Nikolaj Moll, Reto Schlittler, Gerhard Meyer, Fabian Mohn and Leo Gross (left to right) standing behind a scanning tunneling/atomic force microscope. Photo taken by Michael Lowry.*
Dr. Pauling was then able to predict molecular structures and properties for atoms. The new IBM capability in 2009 would have positively meant sweet happiness to him.
Two and three, Gerd Binnig and Heinrich Rohrer, who shared one half of 1986 Nobel Prize in Physics for their design of the scanning tunnelling microscope (STM), worked at IBM Research Laboratory in Zurich, Switzerland. This is also the same laboratory where the scientists (see above) imaged the structure of a molecule. Leo Gross et al., IBM says, "for the first time ever, were able to look through the electron cloud and see the atomic backbone of an individual molecule" with their atomic force microscope (AFM). Moreover, IBM says the AFM, "an offspring of the STM, was invented by Binnig in 1986."
An elegant molecule is that of pentacene.


Left: Textbook model: A ball-and-stick model of the pentacene molecule showing the arrangement of the atoms and the bonds in between. Twenty-two carbon atoms (gray balls) form five interconnected hexagonal rings. Fourteen hydrogen atoms (white balls) bind to the carbon atoms. The molecule itself measures 1.4 nanometers in length.*
Right: The delicate inner structure of a pentacene molecule imaged with an atomic force microscope. For the first time, scientists achieved a resolution that revealed the chemical structure of a molecule. The hexagonal shapes of the five carbon rings in the pentacene molecule are clearly resolved. Even the positions of the hydrogen atoms around the carbon rings can be deduced from the image. (Pixels correspond to actual data points)*[Historic image]
In the historic image above, a pentacene molecule is surrounded by a beautiful dark halo. The carbon-hydrogen bonds are evidently indicating the positions of the 14 hydrogen atoms within the pentacene molecule. To obtain this image, the scientists employed a CO molecule which adsorbed with carbon atom toward the tip as shown in the image below (right). The scientists observed that CO molecule slightly affected the STM image; faint maxima and minima were still visible because of the interaction of the CO with the pentacene orbitals.
Left: A topography of forces: The forces exerted on the tip above the pentacene molecule create a landscape that resembles a mountain ridge in this three-dimensional force map. The overall elevation represents the attractive forces between the tip and the molecule. The finer features on the ridge stem from repulsive forces between the tip and the pentacene molecule at closer tip–molecule distance.*
Right: Imaging the "anatomy" of a pentacene molecule - 3D rendered view: By using an atomically sharp metal tip terminated with a carbon monoxide molecule, IBM scientists were able to measure in the short-range regime of forces which allowed them to obtain an image of the inner structure of the molecule. The colored surface represents experimental data.*
The image above on the left is the 3D map obtained by measuring the forces between CO and pentacene (C22H14) molecules as described in the image on the right. To appreciate the present accomplishment of the IBM scientists even further, I include the topology of the frontier orbitals, HOMO (top) and LUMO (bottom), of pentacene (see below) as calculated by Fabio Pichierri in 2006.
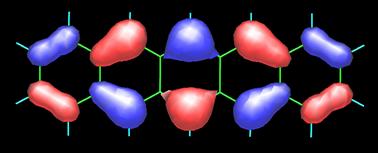
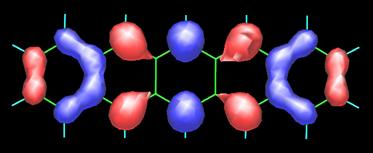
Top-Bottom: HOMO (highest occupied molecular orbital) and LUMO ( lowest occupied molecular orbital) by Fabio Pichierri, Tohoku University, Japan.
The taste of honey will endure as the current research opens new vistas in the nanoworld. Investigations into molecules and molecular networks at atomic scale might lead to "building smaller, faster, and more energy-efficient computing components than today’s processors and memory devices." Pentacene has received attention in recent years because of its behavior as an organic semiconductor for electronic devices.
Credit: Wikipedia
Notes:
*http://www.flickr.com/photos/ibm_research_zurich/sets/72157622092395070/detail
http://www.zurich.ibm.com/news/09/pentacene.html
*http://www.flickr.com/photos/ibm_research_zurich/sets/72157622092395070/detail
http://www.zurich.ibm.com/news/09/pentacene.html
L. Gross, F. Mohn, N. Moll, P. Liljeroth, G. Meyer, The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy, Science 325, 1110 (2009).
Pentacene by Fabio Pichierri, Tohoku University, Japan (2006).









Comments