NASHVILLE, Tenn. – To develop correctly, baby hearts need rhythm...even before they have blood to pump.
"We have discovered that mechanical forces are important when making baby hearts," said Mary Kathryn Sewell-Loftin, a Vanderbilt graduate student working with a team of Vanderbilt engineers, scientists and clinicians attempting to grow replacement heart valves from a patient's own cells.
In an article published last month in the journal Biomaterials the team reported that they have taken an important step toward this goal by determining that the mechanical forces generated by the rhythmic expansion and contraction of cardiac muscle cells play an active role in the initial stage of heart valve formation.
A heart valve is a marvelous device. It consists of two or three flaps, called leaflets, which open and close to control the flow of blood through the heart. It is designed well enough to cycle two to three billion times in a person's lifetime. (Humans and chickens are outliers: Most other animals, large and small, have hearts that beat about one billion times in their lives.) However, heart valves can be damaged by diseases such as rheumatic fever and cancer, aging, heart attacks and birth defects.
"For the last 15 years, people have been trying to create a heart valve out of artificial tissue using brute-force engineering methods without any success," said Assistant Professor of Biomedical Engineering W. David Merryman. "We decided to take a step back and study how heart valves develop naturally so we can figure out how to duplicate the process." To do so, they designed a series of experiments with chickens, whose hearts develop in a fashion similar to the human heart.
"The discovery that the deformations produced by the beating cardiac muscle cells are important provides an entirely new perspective on the process," said Merryman, who directed the three-year study.
The Vanderbilt effort is part of a broader program to develop artificial organs named the Systems-based Consortium for Organ Design and Engineering (SysCODE). It is a National Institutes of Health "Roadmap" initiative to speed the movement of scientific discoveries from the bench to the bedside.
"This is the second major advance that we've made," said Professor of Pharmacology Joey Barnett, co-principal investigator of the heart valve project.
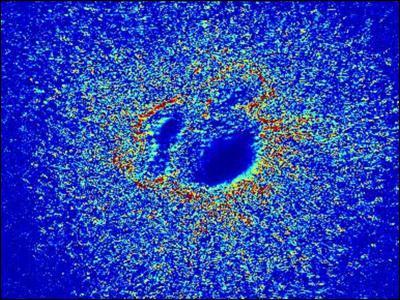
This is a heat map of the valve-formation section of a chick heart sitting on a special hydrogel which is deformed by the beating of the attached heart muscle. Regions of high deformation are colored orange and red while areas of low deformation are colored blue.
(Photo Credit: MK Sewell-Loftin / Vanderbilt)
Last spring, the Vanderbilt team announced that they had identified the unique genes and molecular pathways associated with valve formation. "These included both genes and pathways that we knew about and several that were previously unknown," said Barnett, who has studied heart valves for more than 20 years.
"The genetic study gave us the list of the basic parts – the hardware – required to build a heart valve and this latest study provides us with the information we need about the environment that is required," said the biologist. "With this information, we should have what we need to create valvular interstitial cells (VICs), that are the basic building blocks of heart valves."
The heart starts out as a simple, U-shaped tube of tissue. (In the case of the chicken embryo, it is about the size of a comma on the printed page.) The tube has three layers. The outer layer is made up of cardiac muscle cells that begin pulsing before blood vessels form and attach to the heart. The inner layer consists of specialized endothelial cells, the type of cells that line the interior of blood vessels. Sandwiched between the two is a layer of a complex gelatinous material called cardiac jelly.
At the locations of the inflow and outflow valves, the walls of the tube thicken to form "cushions" of cardiac jelly. After the cushions are formed, the endothelial cells in the region embed themselves in the cushion and transform into VICs. The VICs, in turn, begin guiding the process that transforms the cardiac jelly in the cushion into valve leaflets.
One of the standard laboratory methods for studying the early stages of heart development is to use microsurgery to remove a chick heart from an embryo and place it in a cell-culture dish filled with collagen gel. However, the method was not suitable for studying mechanical forces so Sewell-Loftin had to modify it substantially. She found one key was to include a complex sugar called hyaluronic acid, which is found in cardiac jelly.
Next, she had to devise a method to measure the amount of deformation that the pulsation of the heart muscle cells causes in the gel. She did so by creating a computer program that analyzed sequences of microscope images of the gel surface to estimate the forces caused by the pulsing cells.
When Sewell-Loftin compared her maps with the locations where VICs were being formed, she found that cells were transforming preferentially in areas of high strain.
The team's next step is to collaborate with a researcher who works with induced pluripotent stem cells – a type of stem cell that can be generated directly from adult cells – to produce endothelial cells. Once they have these cells, they hope to produce human VICs. In addition to guiding the initial formation of the heart, VICs are known to play a role in maintaining valve health in adults. So they could provide a better way to repair calcified heart valves, the major cause of open-heart surgery in adults, the researchers speculate.
Once they can make human VICs, there is a good chance that they will create artificial human heart valves when they are placed in a properly designed bioreactor, the researchers anticipate. And once they have artificial human heart valves, they could be used to replace defective valves when needed in the 40,000 babies born with congenital heart defects each year. Hopefully, these artificial valves would grow with the child. Current replacement valves are made out of plastic so they do not grow with a child. That means these young patients must endure multiple surgeries, which multiplies their risk of harmful complications.




Comments