Testosterone has control over the gender-specific absence or presence of mammary gland nerves that sense the amount of milk available in breast milk ducts, according to a new paper which says that the hormones do the job by altering the availability of a nerve growth factor, called BDNF for short.
For their experiments with sex-specific neural wiring, Yin Liu, a student in Ginty's laboratory, studied nerves in mice that monitor the fullness of milk ducts in females. If the milk supply is low, the nerves are believed to report this to the brain to stimulate milk production, Ginty says. Early in embryonic development, there are no differences between the mammary glands of males and females and this milk-monitoring set of nerves is present in both. Later in development, the nerves are lost in males.
In one experiment, to figure out how the nerves find their way to the immature mammary glands of both sexes during early development, the researchers analyzed the gland cells for the presence of four proteins known to encourage nerve growth. They found only one that was there in significant quantities — BDNF — and it was present at similar levels in both sexes.
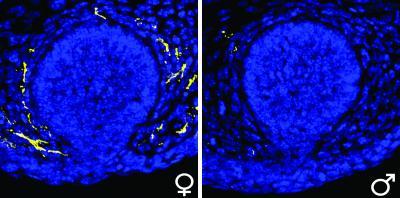
Immature mammary glands from a female (left) and male (right) mouse after the nerve cells (yellow) have retracted from the male tissue. Mammary gland cells labeled in blue. Credit: Yin Liu
"We now think we have a broader understanding of how sex-specific nerves reach their proper target in a given sex, say in mammary milk ducts in females, but disappear in the other sex," says David Ginty, Ph.D., a Howard Hughes investigator and professor of neuroscience in the Institute for Basic Biomedical Sciences at the Johns Hopkins School of Medicine.
Ginty says BDNF is known to bind to a protein, known as TrkB, found on the surface of nerve cells. This binding event triggers a series of messages within the nerve cell, telling it to grow towards the source of the signal. When the researchers looked for TrkB in developing mice, they found it, as expected, on the surfaces of nerve cells that grow into the immature glands of both sexes.
"So early development could be explained quite simply," says Ginty. "The cells of the early mammary glands released the signaling molecule BDNF, which was detected by TrkB on the nerve cells, which made them grow toward the mammary glands. What remained a mystery was why these nerve cells are lost a few hours later in males."
In a subsequent experiment, using molecular tests, the scientists ruled out the possibility that nerve cells in male pre-mammary glands were receiving a "suicide signal" and dying off. They reasoned that if the nerve cells weren't dying, they must be retracting, and went hunting for what signal was telling them to do so.
Since sex hormones play many different roles in determining sex-specific differences, the researchers monitored the effects of adding male sex hormones to females, and the effects of blocking male sex hormones in males. They found that the female pattern of nerve growth was the "default" and that male sex hormones would cause withdrawal of the nerves from the glands of either sex.
"At this point, we knew that BDNF is found at comparable levels in the glands of both sexes, that TrkB is found at comparable levels on the nerve cells of both sexes, and that male sex hormones were still somehow creating a difference in the system," says Ginty.
To figure out just how the hormones caused the nerve growth differences, they searched for the BDNF receptor protein TrkB in the immature gland tissue (instead of in the nerve cells). They found it — but only in males.
It turns out that, in addition to regular TrkB made by nerve cells, a shorter version of the protein, dubbed TrkB.T1, also exists. From their experiments, Ginty and his team concluded that as the male embryos got older, male sex hormones, produced by the testes, commanded non-nerve cells in the immature gland tissue to produce TrkB.T1. Though TrkB.T1 can still bind BDNF, once it does so, both proteins are taken inside the cell and recycled, essentially removing BDNF — and its nerve-growth-promoting signals — from early mammary gland tissue in males.
"It's as if testosterone sounds the horn for retreat, so that without BDNF present, the nerve endings that had already reached the male mammary glands pull away," says Ginty. "We believe this is the first study to show sex hormones regulating nerve growth and retraction by affecting the availability of BDNF," he adds, noting that "it will be interesting to see if similar mechanisms create other sex-specific differences in neural wiring, including those that affect general behavior."
Published in Science.




Comments