Engineers have harnessed the molecular machinery of living systems to power an integrated circuit from adenosine triphosphate (ATP), the energy currency of life, and they did it by integrating a conventional solid-state complementary metal-oxide-semiconductor (CMOS) integrated circuit with an artificial lipid bilayer membrane containing ATP-powered ion pumps, opening the door to creating entirely new artificial systems that contain both biological and solid-state components.
In living systems, energy is stored in potentials across lipid membranes, in this case created through the action of ion pumps. ATP is used to transport energy from where it is generated to where it is consumed in the cell.
To build a prototype of their hybrid system, the team packaged a CMOS integrated circuit (IC) with an ATP-harvesting 'biocell.' In the presence of ATP, the system pumped ions across the membrane, producing an electrical potential harvested by the IC.
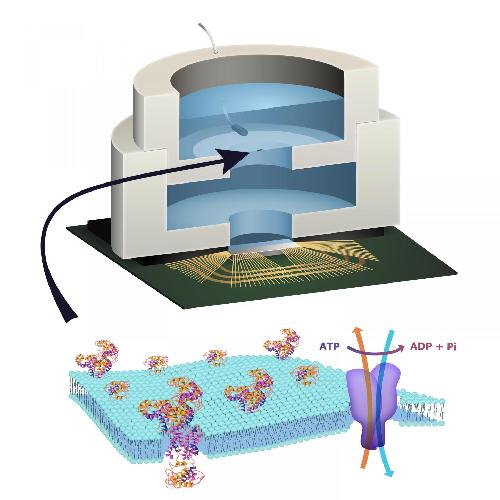
They made a macroscale version of this system, at the scale of several millimeters, to see if it worked. The results provide new insight into a generalized circuit model, enabling them to determine the conditions to maximize the efficiency of harnessing chemical energy through the action of these ion pumps. Then they have to learn how to scale the system down. As is, CMOS solid-state electronics is incapable of replicating certain functions natural to living systems, such as the senses of taste and smell and the use of biochemical energy sources. Living systems achieve this functionality with their own version of electronics based on lipid membranes and ion channels and pumps, which act as a kind of 'biological transistor.'
They use charge in the form of ions to carry energy and information -- ion channels control the flow of ions across cell membranes. Solid-state systems, such as those in computers and communication devices, use electrons; their electronic signaling and power are controlled by field-effect transistors.
While other groups have harvested energy from living systems, Ken Shepard, professor of engineering at Columbia University, and his team are exploring how to do this at the molecular level, isolating just the desired function and interfacing this with electronics. "We don't need the whole cell," he explains. "We just grab the component of the cell that's doing what we want. For this project, we isolated the ATPases because they were the proteins that allowed us to extract energy from ATP."
The ability to build a system that combines the power of solid-state electronics with the capabilities of biological components has great promise. "You need a bomb-sniffing dog now, but if you can take just the part of the dog that is useful -- the molecules that are doing the sensing -- we wouldn't need the whole animal," says Shepard.




Comments