A "structure-based" approach to drug design has led to identification of compounds with the potential to delay or treat Alzheimer's disease, and possibly Parkinson's, Lou Gehrig's disease and other degenerative disorders.
Structure-based drug design, in which the physical structure of a targeted protein is used to help identify compounds that will interact with it, has already been used to generate therapeutic agents for a number of infectious and metabolic diseases.
Degenerative brain disorders such as the ones listed above are marked by harmful, elongated, rope-like structures known as amyloid fibrils, linked protein molecules that form in the brains of patients. An estimated 5 million patients in the U.S. suffer from Alzheimer's disease, the most common form of dementia. Alzheimer's health care costs in have been estimated to be as high as $178 billion per year, including the value of unpaid care for patients provided by nearly 10 million family members and friends.
A number of non-structural screening attempts have been made to identify natural and synthetic compounds that might prevent the aggregation and toxicity of amyloid fibrils. Such studies have revealed that polyphenols, naturally occurring compounds found in green tea and in the spice turmeric, can inhibit the formation of amyloid fibrils. In addition, several dyes have been found to reduce amyloid's toxic effects, although significant side effects prevent them from being used as drugs.
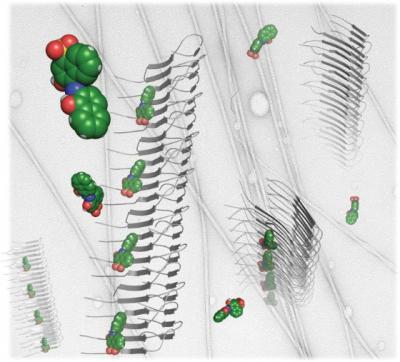
The stacked gray arrows are a artistic representations of the amyloid proteins associated with numerous diseases, including Alzheimer's. When proteins enter amyloid, they stack up like the rungs of a ladder and interfere with normal operations of biological cells. The green and red molecules are atomic representations of one of the compounds discovered by Jiang, et al., which bind to amyloid fibers. Carbon atoms are colored green, oxygen atoms are red, and nitrogen atoms are blue. Credit: Lin Jiang, David Eisenberg/UCLA, Howard Hughes Medical Institute
Armed with a precise knowledge of the atomic structure of the amyloid-beta protein, the researchers conducted a computational screening of 18,000 compounds in search of those most likely to bind tightly and effectively to the protein.
Those compounds that showed the strongest potential for binding were then tested for their efficacy in blocking the aggregation of amyloid-beta and for their ability to protect mammalian cells grown in culture from the protein's toxic effects, which in the past has proved very difficult. Ultimately, the researchers identified eight compounds and three compound derivatives that had a significant effect.
While these compounds did not reduce the amount of protein aggregates, they were found to reduce the protein's toxicity and to increase the stability of amyloid fibrils — a finding that lends further evidence to the theory that smaller assemblies of amyloid-beta known as oligomers, and not the fibrils themselves, are the toxic agents responsible for Alzheimer's symptoms.
The researchers hypothesize that by binding snugly to the protein, the compounds they identified may be preventing these smaller oligomers from breaking free of the amyloid-beta fibrils, thus keeping toxicity in check.
In addition to uncovering compounds with therapeutic potential for Alzheimer's disease, this research presents a new approach for identifying proteins that bind to amyloid fibrils — an approach that could have broad applications for treating many diseases.
Citation: L Jiang, C Liu, D Leibly, M Landau, M Zhao, MP Hughes, DS Eisenberg, 'Correction: Structure-based discovery of fiber-binding compounds that reduce the cytotoxicity of amyloid beta', eLife July 23rd, 2013 DOI:10.7554/eLife.01252




Comments