Traditionally, to understand how a gene functions, a scientist would breed an organism that lacks that gene - "knocking it out" - then ask how the organism has changed. Are its senses affected? Its behavior? Can it even survive? Thanks to the recent advance of gene editing technology, this gold standard genetic experiment has become much more accessible in a wide variety of organisms. Now, researchers at Rockefeller University have harnessed a technique known as CRISPR-Cas9 editing in an important and understudied species: the mosquito, Aedes aegypti, which infects hundreds of millions of people annually with the deadly diseases chikungunya, yellow fever, and dengue fever.
Researchers led by postdoctoral fellow Benjamin J. Matthews adapted the CRISPR-Cas9 system to Ae. aegypti and were able to efficiently generate targeted mutations and insertions in a number of genes. The immediate goal of this project, says Matthews, is to learn more about how different genes help the species operate so efficiently as a disease vector, and create new ways to control it. "To understand how the female mosquito actually transmits disease," says Matthews, "you have to learn how she finds humans to bite, and how she chooses a source of water to lay her eggs. Once you have that information, techniques for intervention will come."
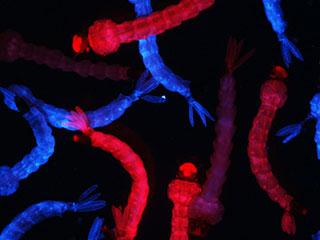
Mosquito larvae from two different lines fluoresce in different colors thanks to genetic tags that were inserted using the CRISPR-Cas9 gene editing system. Credit: Vosshall Laboratory
In the study, published March 26 in Cell Reports, Matthews and research assistant Kathryn E. Kistler, both in Leslie B. Vosshall's Laboratory of Neurogenetics and Behavior, adapted the CRISPR-Cas9 system to introduce precise mutations in Ae. aegypti. Previously, to create these types of mutations, scientists relied on techniques that used engineered proteins to bind to specific segments of DNA they wanted to remove, a process that was both expensive and unreliable. CRISPR-Cas9, in contrast, consists of short stretches of RNA that bind to specific regions of the genome where a protein, Cas9, cleaves the DNA. Scientists have been studying how RNA binds to DNA for decades and "the targeting is done with rules that we have a good handle on," says Matthews, which makes it easy to reprogram CRISPR-Cas9 to target any gene.
"This amazing technique has worked in nearly every organism that's been tried," says Vosshall, who is Robin Chemers Neustein Professor and a Howard Hughes Medical Institute investigator. "There are lots of interesting animal species out there that could not be studied using genetics prior to CRISPR-Cas9, and as a result this technique is already revolutionizing biology."
This work opens the door to learning more about the role of specific genes the Vosshall lab suspects may help mosquitoes propagate, perhaps by finding the perfect spot to lay their eggs. Their protocols will likely also help other scientists apply the same technique to study additional organisms, such as agricultural pests or mosquitoes that carry malaria.
"Before starting this project, we thought it would be difficult to modify many genes in the mosquito genome in a lab setting" Matthews says. "With a little tweaking, we were able to make this technique routine and it's only going to get easier, faster, and cheaper from here on out."





Comments