Exposure to cold temperatures is known to mimic the effects of exercise, protecting against obesity and improving metabolic health. A study published December 3 in Cell now reveals that the beneficial health effects of cold exposure are mediated in part by gut microbes. The researchers found that cold exposure dramatically alters the composition of intestinal bacteria in mice and that this microbial shift is sufficient to burn fat, improve glucose metabolism, and reduce body weight.
"We provide compelling evidence that gut microbes play a key role in our ability to adapt to the environment by directly regulating our energy balance," says senior study author Mirko Trajkovski of the University of Geneva. "We are excited about exploring the therapeutic potential of these findings and testing whether targeting some of these microbes could be a promising approach for preventing obesity and related metabolic conditions."
One potential therapeutic avenue for obesity centers on promoting the formation of good types of body fat known as brown and beige fat. Human infants have large amounts of heat-generating brown fat to protect them from extreme cold, and scientists recently discovered that adult humans also retain brown fat stores consisting mainly of a subtype known as beige fat. Cold exposure or exercise can promote the formation of beige fat, thereby burning stored calories and protecting mammals from hypothermia, obesity, and metabolic problems.
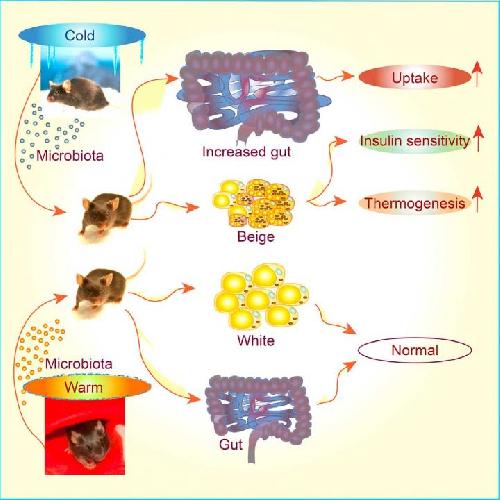
Because gut microbes have been implicated in obesity and related metabolic conditions, Trajkovski and his team suspected that they might also play a role in mediating the positive health effects of cold exposure. In support of this idea, they found that exposure to a cold temperature (6° Celsius, 43° Fahrenheit) for up to 10 days caused a major shift in the composition of gut microbes while preventing weight gain in mice.
The researchers next tested the direct impact of these microbes on metabolic health. To do so, they transplanted the cold-induced gut bacteria into other mice that did not harbor gut microbes because they had been raised in a germ-free environment. The transplanted microbes improved glucose metabolism, increased tolerance to cold temperatures, and caused weight loss in the recipient mice by promoting the formation of beige fat. "These findings demonstrate that gut microbes directly regulate the energy balance in response to changes in the environment," Trajkovski says.
However, after three weeks of cold exposure, body weight began to stabilize. The researchers suspected that the intestine was absorbing more nutrients from food, counteracting additional weight loss that would otherwise result from higher overall energy expenditure.
In support of this idea, transplantation experiments showed that gut microbes associated with long-term cold exposure caused the intestine to grow in size and triggered an increase in the surface area of intestinal cells that absorb nutrients. "These findings demonstrate that gut microbes enable mammals to harvest more energy from food as a way to adapt to the increased energy demand associated with long periods of cold exposure, thereby helping to protect against hypothermia," Trajkovski says. "We were surprised to see that gut microbes had such dramatic effects on the structure and function of the intestine."
Moving forward, the researchers plan to study the molecular mechanisms by which gut microbes sense changes in the environment to affect the energy balance of the host. Another avenue of investigation centers on the idea that certain bacteria may prevent obesity by remodeling intestinal tissue and thereby decreasing the absorption of nutrients in the gut.




Comments