Genome editing using CRISPR/Cas system has enabled direct modification of the mouse genome in fertilized mouse eggs, leading to rapid, convenient, and efficient one-step production of knockout mice without embryonic stem cells.
In contrast to the ease of targeted gene deletion, the complementary application, called targeted gene cassette insertion or knock-in, in fertilized mouse eggs by CRISPR/Cas mediated genome editing still remains a tough challenge.
A research group overcame this issue by developing innovative highly efficient CRISPR/Cas system, which resulted in targeted insertion of long gene cassette including enhanced green fluorescent protein (EGFP) into mouse genome in fertilized eggs with efficiency up to approx. 50%. The team reproduced the natural state of CRISPR/Cas system, which consists of three components: Cas9 protein, CRISPR RNA (crRNA), and trans activating crRNA (crRNA), instead of commonly used two-component system which consists of Cas9 mRNA and single guide RNA (sgRNA), leading to extremely high efficiency.
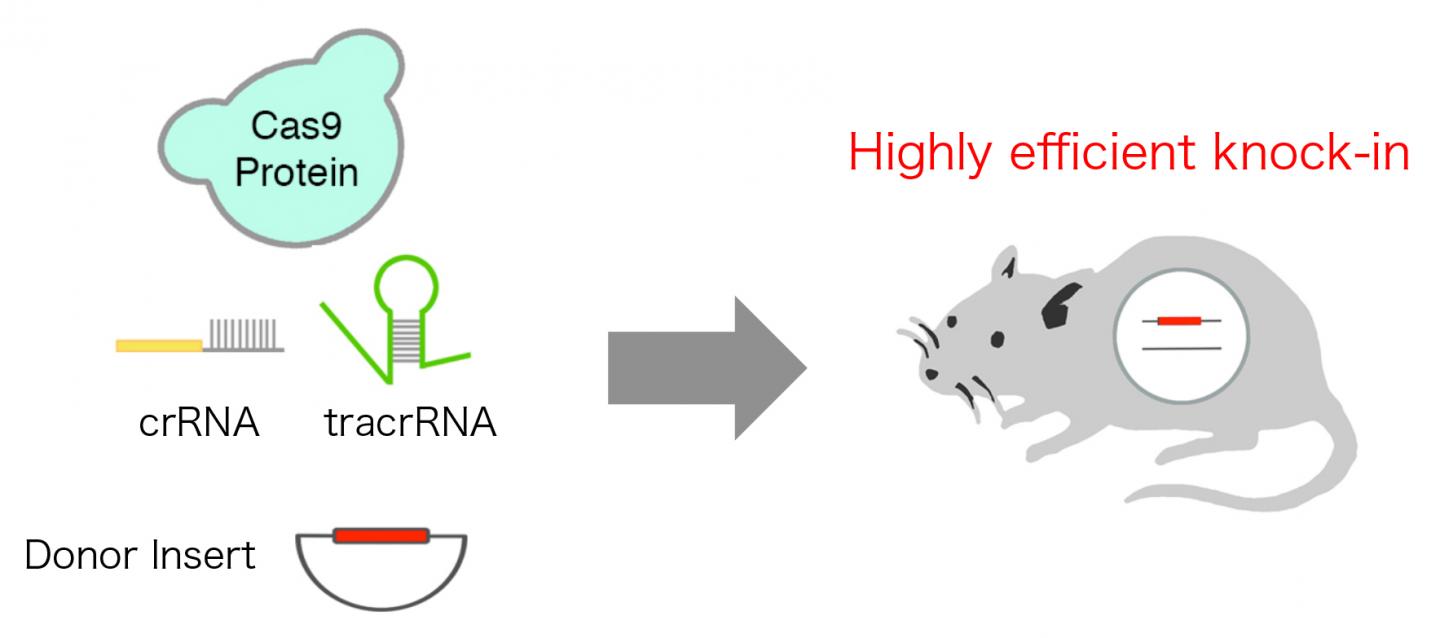
A schematic diagram of the highly efficient CRISPR/Cas system, which leads to super efficient targeted insertion (knock-in) of a long donor insert into mouse genome. Credit: TMDU
The improved CRISPR/Cas system further provides highly convenient and accurate gene modification, and its successful transmission to the next generations.
This improved CRISPR/Cas system will be useful for a variety of applications, including creation of humanized mice for modeling of genetic diseases, drug metabolisms, immunity, and infectious diseases. Further, accurate targeted insertion will improve the safety of gene therapy in human patients in the future. The new system can be also applied to other purposes such as production of livestock, fishes, plants, and microorganisms carrying useful traits.
Citation: "Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice", Genome Biology, April 29, 2015.




Comments