3 million men across America experience infertility. Today, researchers described a key event during sperm development that is essential for male fertility - a protein controls DNA packaging to protect a man's genetic information.
The sperm is a simple delivery vehicle for a man's genetic information. The highly specialized cell is little more than a DNA bundle powered by molecular motors. As such, it is necessarily tiny: from head to tail a sperm cell is only about 50 micrometers long (1/500th of an inch), invisible to the naked eye.
An egg is 30 times larger. The sperm's small size has its benefits – less bulk to carry while searching for an egg – but it also presents significant challenges. A man's genetic material must be very tightly packaged to fit within a minuscule space.
This organizational problem is not unique to sperm. Every cell in our body contains a full human genome, which spans nearly two meters (6 feet) if unfurled. To contain this massive length of DNA, cells tightly compress our genetic information. In every cell nucleus, DNA is wrapped like thread around protein spools, called histones.
The thread can be easily unwound at any time to allow access to the genetic information. In sperm, the packaging problem is much more acute, as its DNA is even more condensed. The spool-like histones are replaced with tiny proteins called protamines. This repackaging process, called chromatin remodeling, is absolutely essential for male fertility.
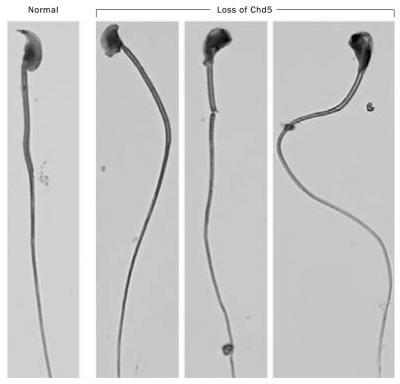
Researchers have discovered that a protein, called Chd5, plays an important role in male fertility. On the left is a sperm cell from a mouse that has two normal copies of the Chd5 gene. The sperm is healthy and the mouse is fertile. In the absence of Chd5, mice are infertile and their sperm cells are misshapen (three examples on the right). The researchers discovered that loss of Chd5 disrupts DNA packaging which causes infertility. Credit: Alea Mills, Cold Spring Harbor Laboratory
In work published today in Nature Communications, a team led by Cold Spring Harbor Laboratory Professor Alea Mills explains how
and her team identify a protein, called Chd5, as a key regulator of chromatin remodeling during sperm development. Mills and Wangzhi Li, PhD, lead author on the study, removed both copies of the Chd5 gene from male mice. They discovered that these males had severe fertility defects, ranging from low sperm counts to decreased sperm motility. The defective sperm failed to fertilize eggs when in vitro fertilization (IVF) was performed.
Mills has been interested in Chd5 since the time that her team first discovered it in 2007 as a potent tumor suppressor, one that can stop cells from becoming cancerous. "We know this ability has something to do with chromatin remodeling -- that when defective, causes normal cells to transform into tumors," says Mills. "But the most dramatic chromatin reorganization occurs when specialized cells carrying our genetic blueprint develop into sperm cells. It makes sense that Chd5 would be functioning there, too."
This, indeed, is what Mills and her team found. When Chd5 is missing, chromatin remodeling is disrupted. Histones are not efficiently replaced with protamines to repackage DNA, resulting in a more uneven, less condensed genome.
This change in DNA packaging has dramatic effects on the DNA itself. In the absence of Chd5, the double helix becomes damaged, breaking at multiple points throughout the genome. "So in addition to infertility, loss of Chd5 may put future generations – the rare embryos that do get fertilized with defective sperm – at risk for disease," says Mills. "Chd5 may protect a person from medical conditions related to DNA damage and spontaneous mutations, like cancer and autism."
The team is actively studying the role of Chd5 in human fertility. They analyzed Chd5 levels using data from testes biopsies obtained from men with fertility defects. "We found that men with more severe defects had the lowest levels of Chd5," says Mills. "While it is only a correlation at this point, we are eager to understand fully how Chd5 affects sperm development in humans."




Comments