The difficulty in subduing the pandemic strain of drug-resistant E. coli, called H30, may go beyond patient vulnerability or antibiotic resistance. This form of the disease-pathogen may have an intrinsic ability to cause persistent, harmful, even deadly infections.
The bacterium E. coli comes in many different varieties. Many strains live unobtrusively in the gut or innocuously in the environment. Some strains can cause diarrhea. Others can invade the urinary tract, the blood stream, or other parts of the body to provoke varying degrees of illness, from mild to serious and sometimes fatal.
A particular genetic family of E. coli, Sequence Type 131, produced the H30 strain, which appeared in late 1990s. In an almost unprecedented show of microbial force, it expanded around the world to become the dominant drug-resistant strain in virtually all populations.
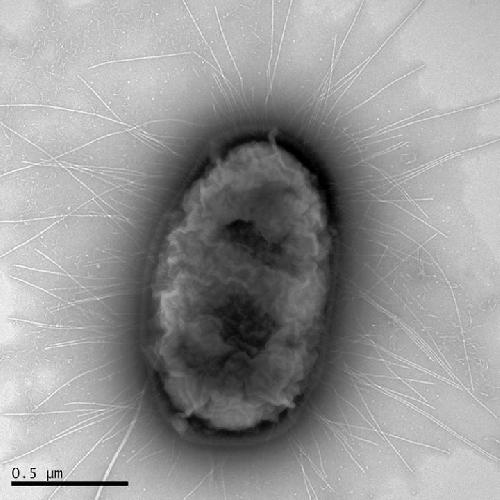
"No other type of E. coli is causing this much widespread damage," said Evgeni Sokurenko, University of Washington professor of microbiology. "We need to pay as much attention to it as we do to the superbug MRSA, the treatment-resistant staph infection."
To make matters worse, H30 begins as a subtle infection that is hard to detect, he explained.
Once diagnosed, even if the right antibiotic is given, physicians have trouble eradicating the infection, and complications can set in.
The power of the H30 strain, and why it has become so common, is puzzling. According to Sokurenko, most animal studies have failed to confirm that the H30 strain is especially virulent. It has also been assumed that H30 causes opportunistic infections, by taking advantage of the rapidly growing elderly population and others with weakened immune systems.
It is also thought that broad-spectrum antibiotic use might have promoted H30's dominance by making it resistant to several drugs, particularly the fluoroquinolones (such as Cipro), which are widely prescribed for urinary tract infections.
These factors, as well as possible presence of as yet-unknown pathogen traits, could have contributed to H30's emergence as a public health problem. Each year in the United States alone, H30 may cause more than 1 million urinary tract infections, as well as much more lethal blood infections.
Sokurenko is one of the leaders of a team of researchers from the University of Washington, the Minneapolis Veterans Affairs Health Care System, the University of Minnesota, Seattle Children's Hospital and Group Health Cooperative to assess the nature of H30 infections.
Analyzing epidemiological and medical data, the team explored possible associations of H30 with patient characteristics, clinical manifestations, treatment, and how well or poorly the patients fared.
Their findings are published in the journal, Clinical Infectious Diseases. James R. Johnson, staff physician and director of the Molecular Epidemiology Unit at the Minneapolis VA Health Care System, and professor of medicine at the University of Minnesota, was the lead author of the study.
The researchers found that individuals at greater risk for E. coli H30 infection tended to be older women and men who had been in a healthcare facility, including long term care residences or hospitals, who had received antibiotics, and who had underlying conditions that weakened their ability to ward off infections.
The researchers also observed that patients who were later shown to have H30 were, at their first visit with the doctor, significantly less likely to be suspected of having an infection, and less likely to receive a proper antibiotic prescription.
Within a month afterward, patients whose initial strain was H30 were more likely to experience a severe complication, including a hospital admission or a new infection at a different site.
Thus, the researchers reported that H30 "was strongly associated with ineffective initial antimicrobial therapy, clinical and microbial persistence, and diverse, later-occurring adverse effects."
And even when the H30 infection seemed like any other E. coli infection during the first clinical encounter, and the patient received a correct antibiotic, the infection could persist for an unusually long time.
"We don't know the exact reason, but it's possible that H30 might have been hiding from the patients' natural defenses against infection, thereby impairing the body's attempt to clear the pathogen during treatment," Sokurenko said.
"Distinctive properties that perhaps allow H30 to act as a defenses-evading pathogen might also be associated with the delayed complications," he surmised.
He added that the basis for the lack of response to supposedly effective antibiotic treatment and reasons for later-appearing complications is still unclear.
"H30 might have that dangerous combination of being both highly resistant to antibiotics and highly successful as a stealth pathogen," Sokurenko said. "This double-trouble may be why H30 is so widespread and has become a superbug. What makes it worse is that can go unnoticed in a patient until it causes significant damage."
Sokurenko would like to see better ways to anticipate, detect, and diagnose H30 in the elderly or other vulnerable patients who don't have the usual symptoms of urinary tract or bloodstream infection.
In addition to improved surveillance, he also would like rapid tests to become available to determine which antibiotics may or may not work for an individual patient, to avoid antibiotic-bacteria mismatch and delayed treatment response.
"Our findings reinforce the need for a more 'individualized medicine' approach to prescribing antibiotics for E. coli infections," he said. "Also, even if the infection seems mild the patient should be monitored carefully so it does not progress unchecked."
The other authors of the study were Paul Thuras, Brian D. Johnston, Scott J. Weissman, Ajit P. Limaye, Kim Riddell, Delia Scholes, and Veronika Tchesnokova.
Three of the researchers have patent applications pending for tests for E. coli H30 strain. Sokurenko also is a founder and a major shareholder in ID Genomics.




Comments