The basics of how a muscle generates power are this: Filaments of myosin tugging on filaments of actin shorten, or contract, the muscle. Since the 1950s physiologists have had a formula – the length-tension curve – that accurately describes the force a muscle exerts at all points from fully outstretched, when every weight lifter knows there is little strength, to the middle points that display the greatest force, to the completely shortened muscle when, again, strength is minimized.
The assumption for 50 years has been that the power comes primarily from what's happening straight up and down the length of the muscle.
Not so, according to a new paper. Instead, as muscles bulge, the filaments are drawn apart from each other, the myosin tugs at sharper angles over greater distances, and it's that action that deserves credit for half the change in muscle force scientists have been measuring.
"The predominant thinking of the last 50 years is that 100 percent of the muscle force comes from changes as muscles shorten and myosin and actin filaments overlap. But when we isolated the effects of filament overlap we only got about half the change in force that physiologists know muscles are capable of producing," said C. David Williams, a postdoctoral researcher at Harvard University and lead author of the study in Proceedings of the Royal Society B.
The rest of the force, he says, should be credited to the lattice work of filaments as it expands outward in bulging muscle – whether in a body builder's buff biceps or the calves of a sinewy marathon runner.
"One of the major discoveries that David Williams brought to light is that force is generated in multiple directions, not just along the long axis of muscle as everyone thinks, but also in the radial direction. This aspect of muscle force generation has flown under the radar for decades and is now becoming a critical feature of our understanding of normal and pathological aspects of muscle," said co-author Thomas Daniel, University of Washington professor of biology and one of Williams' advisers while they did the work.
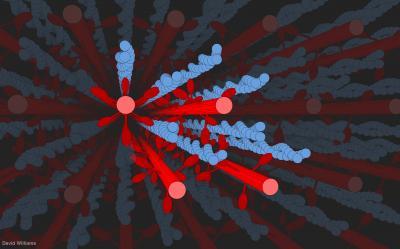
C. David Williams created a 3-D computer model of filaments of myosin (in red) reaching out and tugging along filaments of actin (in blue, looking like stands of pearls twined together) during the contraction of a muscle. The model allowed researchers to consider the geometry and physics at work on the filaments when a muscle bulges. Credit: D Williams/University of Washington
"In the heart especially, because the muscle surrounds the chambers that fill with blood, being able to account for forces that are generated in several directions during muscle contraction allows for much more accurate and realistic study of how pressure is generated to eject blood from the heart," said co-author Michael Regnier, a bioengineering professor at University of Washington. "The radial and long axis forces that are generated may be differentially compromised in cardiac diseases and these new, detailed models allow this to be studied at a molecular level for the first time. They also take us to a new level in testing therapeutic treatments targeted to contractile proteins for both cardiac and skeletal muscle diseases."
Williams developed computer models to consider the geometry and physics at work on the filaments at all those points.
"The ability to model in three dimensions and separate the effects of changes in lattice spacing from changes in muscle length wouldn't even have been possible without the advent of cloud computing in the last 10 years, because it takes ridiculous amounts of computational resources," Williams said.
He and his co-authors validated the force trends using X-ray diffraction and moth flight muscle, which is very similar to human cardiac muscle.

C. David Williams with X-ray diffraction apparatus used to measure lattice spacing of filaments in moth wing muscle samples. Credit: A Kidder
"The fact that lattice spacing changes over the length-tension curve has been known for some time, but its importance in generating the steep length dependence of force has not been previously demonstrated," the co-authors wrote. "This study forms a new basis of interpretation for experiments and thinking about the mechanisms regulating contraction. In the end, we cannot properly understand the length-tension curve unless the three-dimensional geometry . . . is taken into account."
Neither the computational power nor X-ray diffraction facilities have been readily available to other laboratory groups, two reasons Williams thinks this is the first time these findings have been documented. Then too, most muscle research uses "skinned" cells where the membrane of the cell is removed or pierced so it is easier to add or remove calcium to activate or deactivate the actions between myosin and actin.
Skinned cells don't keep constant volume, as occurs in intact muscles, perhaps causing physiologists to overlook changes in geometry that turn out to play a role in force generation, he said.




Comments