The world of "Fantastic Voyage" is rapidly approaching. Tiny devices are being used in therapeutic applications, and development of nanoparticles that can transport and deliver drugs to target cells in the human body is progressing also.
Recently, researchers created nanoparticles that under the right conditions, self-assemble – trapping complementary guest molecules within their structure. Like tiny submarines, these versatile nanocarriers can navigate in the watery environment surrounding cells and transport their guest molecules through the membrane of living cells to sequentially deliver their cargo.
Although the transport of molecules inside cells with nanoparticles has been previously achieved using various methods, researchers have developed nanoparticles capable of delivering and exchanging complementary molecules. For practical applications, these nanocarriers are highly desirable, explains Francisco Raymo, professor of chemistry in the University of Miami College of Arts and Sciences and lead investigator of this project.

The sequential transport of donors and acceptors across cell membranes with independent and dynamic nanocarriers enables energy transfer exclusively in the intracellular space with concomitant fluorescence activation.
Credit: Francisco Raymo, professor of Chemistry and director of the laboratory for molecular photonics, at the University of Miami
"The ability to deliver distinct species inside cells independently and force them to interact, exclusively in the intracellular environment, can evolve into a valuable strategy to activate drugs inside cells," Raymo says.
The new nanocarriers are15 nanometers in diameter. They are supramolecular constructs made up of building blocks called amphiphilic polymers. These nanocarriers hold the guest molecules within the confines of their water-insoluble interior and use their water-soluble exterior to travel through an aqueous environment. As a result, these nanovehicles are ideal for transferring molecules that would otherwise be insoluble in water, across a liquid environment.
"Once inside a living cell, the particles mix and exchange their cargo. This interaction enables the energy transfer between the internalized molecules," says Raymo, director of the UM laboratory for molecular photonics. "If the complementary energy donors and acceptors are loaded separately and sequentially, the transfer of energy between them occurs exclusively within the intracellular space," he says. "As the energy transfer takes place, the acceptors emit a fluorescent signal that can be observed with a microscope."
Essential to this mechanism are the noncovalent bonds that loosely hold the supramolecular constructs together. These weak bonds exist between molecules with complementary shapes and electronic properties. They are responsible for the ability of the supramolecules to assemble spontaneously in liquid environments. Under the right conditions, the reversibility of these weak noncovalent contacts allows the supramolecular constructs to exchange their components as well as their cargo.
The experiments were conducted with cell cultures. It is not yet known if the nanoparticles can actually travel through the bloodstream.
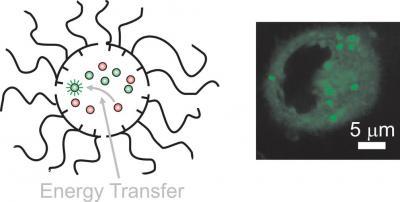
The sequential transport of donors and acceptors across cell membranes with independent and dynamic nanocarriers enables energy transfer exclusively in the intracellular space with concomitant fluorescence activation. Credit: Francisco Raymo, professor of Chemistry and director of the laboratory for molecular photonics, at the University of Miami
"That would be the dream, but we have no evidence that they can actually do so," Raymo says. "However, this is the direction we are heading."
The next phase of this investigation involves demonstrating that this method can be used to do chemical reactions inside cells, instead of energy transfers.
"The size of these nanoparticles, their dynamic character and the fact that the reactions take place under normal biological conditions (at ambient temperature and neutral environment) makes these nanoparticles an ideal vehicle for the controlled activation of therapeutics, directly inside the cells," Raymo says.
The current study is titled "Intracellular guest exchange between dynamic supramolecular hosts." It's published in the Journal of the American Chemical Society. Other authors are John F. Callan, co-corresponding author of the study, from the School of Pharmacy and Pharmaceutical Sciences at the University of Ulster; Subramani Swaminathan and Janet Cusido from the UM's Laboratory for Molecular Photonics, Department of Chemistry in the College of Arts and Sciences; and Colin Fowley and Bridgeen McCuaghan, School of Pharmacy and Pharmaceutical Sciences at the University of Ulster.




Comments