The human body is a cross between a factory and a construction zone -- at least on the cellular level. Certain proteins act as project managers, which direct a wide variety of processes and determine the fate of the cell as a whole.
One group of proteins called the WD-repeat (WDR) family helps a cell choose which of the thousands of possible gene products it should manufacture. These WDR proteins fold into a three-dimensional structure resembling a doughnut -- an unusual shape that allows WDR proteins to act as stable platforms on which large protein complexes can assemble or disassemble.
A new study conducted by scientists at UC Santa Barbara reveals a novel function for WDR5, a protein known for its critical role in gene expression whereby information encoded in genes is converted into products like RNA (ribonucleic acid) and protein. In cells, WDR5 is a subunit of a five-protein complex. Mutations in members of this complex can result in childhood leukemia and other disorders affecting numerous organ systems in the body. The UCSB team worked with WDR5 in cultured human cell lines.
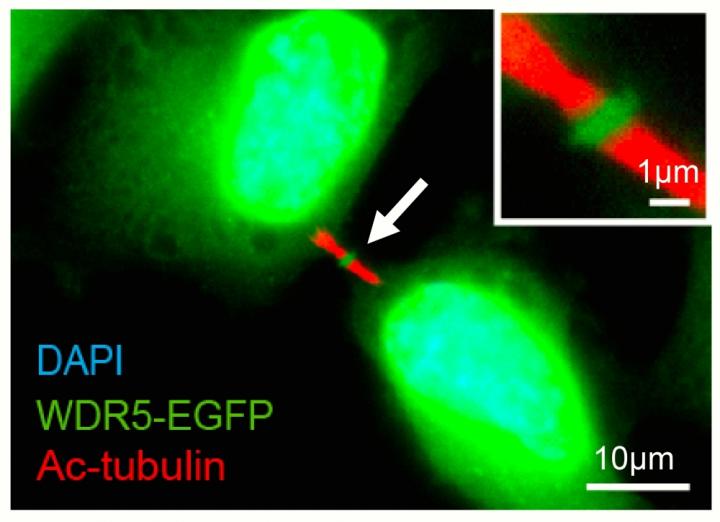
In addition to its location in the cell nucleus, WDR5-EGFP was also found in central dark zone of the midbody. Credit: UCSB
"We found that when two cells divide, WDR5 is localized to a very interesting cellular structure called the midbody," said lead author Jeff Bailey, a graduate student in UCSB's Department of Molecular, Cellular and Developmental Biology (MCDB). "In the past, although associated with cell division, the midbody was considered 'junk,' but that has changed in the last decade. Now the midbody is believed to be important during stem cell differentiation."
When a stem cell divides to produce a differentiated type of cell like a skin cell or a neuron, stem cells retain the midbody while differentiated cells do not. "This suggests that the midbody has important functions," Bailey explained. "Also, when the midbody isn't cut correctly, the cells can re-fuse, creating one cell with two nuclei. This is thought to be part of what happens when a tumor forms."
Conducted in the laboratory of MCDB associate professor Zach Ma, this new work involved the fusion of WDR5 to a green fluorescent protein molecule called EGFP. Although dense material within the midbody thwarts conventional methods of protein detection, the fluorescence of EGFP tethered to WDR5 revealed its location during cell division, or cytokinesis.
The researchers were surprised to find WDR5 in a part of the midbody called the dark zone. "It was very unexpected," Bailey said. "The presence of WDR5 outside the cell nucleus gave us a clue about its function, which we tested," Ma added.
The scientists found that not only did the protein localize in the midbody, it also contributed to abscission, the separation of two daughter cells at the completion of cytokinesis. In addition, WDR5 promotes the disassembly of midbody microtubules, the major structural components of the midbody that must be cleared before abscission can occur.
When the investigators artificially reduced the amount of WDR5 in cells, cytokinesis was substantially delayed and more cells failed to divide properly. "When histology is performed on a tumor, pathologists look for cells that have two nuclei," Bailey explained. "This can indicate that cells within the tumor are failing to properly finish cytokinesis."
Because a single protein can perform several distinct functions according to its location within a cell, it can be challenging to study one function without disrupting the others. Guided by previous structural studies, however, the UCSB team identified surfaces of the WDR5 "doughnut" that may be specific to its role in cell division.
"We have shed some light on the role of WDR5 in cytokinesis," Ma said, "which may in turn help us better understand the diverse array of physiological as well as pathological events related to malfunction of these proteins in the process of cell division."
The results of the study appear in the Journal of Biological Chemistry.





Comments