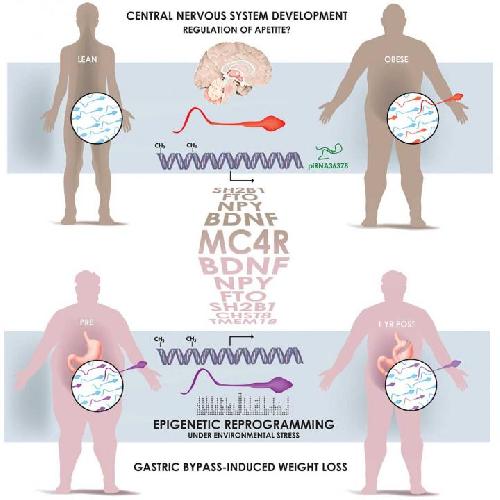
Turns out dads are also eating for two. A study published December 3 in Cell Metabolism reveals that a man's weight affects the heritable information contained in sperm. The sperm cells of lean and obese men possess different epigenetic marks, notable at gene regions associated with the control of appetite. The comparisons, which included 13 lean men and 10 obese men, offer one biological explanation for why children of obese fathers are themselves more predisposed to obesity.
In the next phase of the study, the investigators tracked 6 men undergoing weight-loss surgery to see how it affected their sperm. An average of 5,000 structural changes to sperm cell DNA were observed from the time before the surgery, directly after, and one year later. More needs to be learned about what these differences mean and their effects on offspring, but it is early evidence that sperm carries information about a man's health.
"Our research could lead to changing behavior, particularly pre-conception behavior of the father," says senior author Romain Barrès, an Associate Professor at the University of Copenhagen. "It's common knowledge that when a woman is pregnant she should take care of herself--not drink alcohol, stay away from pollutants, etc.--but if the implication of our study holds true, then recommendations should be directed towards men, too."
Barrès was inspired by a 2005 study showing that the availability of food to people living in a small Swedish village during famine correlated with the risk of their grandchildren developing cardiometabolic diseases (DOI 10.1038/sj.ejhg.5201538). The nutritional stress of the grandparents was likely passed down via epigenetic marks--these can be chemical additions on protein that wrap up DNA, methyl groups that change the structure of DNA once attached, or molecules called small RNAs. Epigenetic marks can control how genes are expressed, and this has also been shown to affect the health of offspring in insects and rodents.
In his study, Barrès and colleagues compared specific epigenetic marks in the ejaculate of lean and obese men (men were the focus because sperm is much easier to obtain than eggs). While no differences were seen in the proteins that wrap up DNA, there were variations between the participants' small RNAs (for which the function is not yet determined) as well as methylation of genes associated with brain development and appetite. The next question was whether these differences were byproducts of obesity or lifestyle, which yielded the look at how bariatric surgery affects sperm epigenetics and discovery that weight is the main factor.
There are likely evolutionary reasons why information about a father's weight would be valuable to offspring. Barrès theory is that in times of abundance, it's an instinctual way to encourage children to eat more and grow bigger. "It's only recently that obesity is not an advantage," he says. "Only decades ago, the ability to store energy was an advantage to resist infections and famines."
To learn more about the epigenetic-offspring connection, his lab is now collaborating with a fertility clinic to study epigenetic differences in discarded embryos generated from the sperm of men with various degrees of body weight. (By law in Denmark, after five years, embryos must be discarded and can be used for research.) Further comparative data will be taken from cord blood of the children that each of the men fathered, but it will take some time to accumulate a large cohort of participants.
"It is clear that these epigenetic changes happen in mice and rats," Barrès says, "but we also need to know if this also happens in humans and whether this is a significant driver for changing our traits."




Comments