Chlamydia trachomatis is a human pathogen that is the leading cause of bacterial sexually transmitted disease worldwide.
More than 90 million new cases of genital infections occur each year. About 70 percent of women infected with Chlamydia remain asymptomatic and these bacteria can establish chronic infections for months and even years. Even when it causes no symptoms, Chlamydia can damage a woman's reproductive organs but standard antibacterial drugs are proving increasingly ineffective in complete eradication, as Chlamydia goes in to persistent mode, leading to asymptomatic chronic infection.
Researchers at the Max Planck Institute for Infection Biology in Berlin (MPIIB) now show that Chlamydia infections can cause mutations in the host DNA by overriding the normal mechanisms by which their host prevents unregulated growth of genetically damaged cells that pave the way for the development of cancer.
Owing to their intracellular lifestyle Chlamydia depend on various host cell functions for their survival. Chlamydia manipulates the host cell mechanism to favour its growth, however the consequences of such alterations on the fate of host cells remains enigmatic. Even more worrying is mounting epidemiological evidence which links Chlamydia infections with the development of cervical and ovarian cancer. Cindrilla Chumduri, Rajendra Kumar Gurumurthy and Thomas F. Meyer, researchers at the Max Planck Institute for Infection Biology in Berlin, have now discovered that Chlamydia induces long-lasting effects on the genome and epi-genome of their host cells. Such changes are increasingly implicated in the development of a range of cancers.
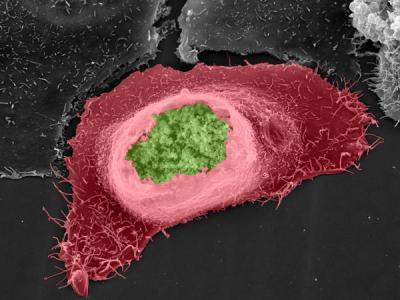
Chlamydia (green) sheltered inside a human host cell (red). Credit: MPI for Infection Biology/V. Brinkmann
The team found increased levels of DNA breaks in Chlamydia-infected cells. In normal cells, depending on the extent of damage, cells either "commit suicide" or activate repair by special protein complexes in a process called the DNA Damage Response, which reseals the broken strands of DNA and makes sure the sequence of the genetic code has not been changed.
Crucially, in Chlamydia-infected cells the DNA Damage Response was impaired, leading to an error-prone repair of the DNA breaks- a potential cause of mutations. Strikingly, despite the presence of extensive DNA damage, Chlamydia infected cells continued to proliferate, facilitated by additional pro-survival signals activated in the host cells by Chlamydia.
The flip-side of this forced survival of damaged cells is an increased tendency to evade the normal mechanisms that eliminate cells carrying mutations that could lead to cancer. The team believe that this could be the first step on the path to carcinogenesis of the infected cells, due to uncontrolled cell growth in the presence of accumulating DNA damage – the hallmark of cancer.
The identification of infections as the origin of human cancers is important since it would allow early prevention of cancerogenesis by means of vaccination or antibiotic treatment. Such preventive strategies are currently successfully pursued against the cancer-inducing agents Human Papiloma Virus (HPV) and Helicobacter pylori, the etiological agents of cervical and gastric cancer, respectively. However, many infection-based cancer etiologies have not been firmly established and therefore cancer treatment is usually restricted to patients at an advanced stage and with an established cancer diagnosis.
The department of Professor Meyer at MPIIB therefore vigorously pursues several lines of research to unequivocally assess the linkage between bacterial infections and cancer, apart from the well-known carcinogenic role of H. pylori. The current paper by Chumduri et al. constitutes one important mosaic piece, corroborating a potential link between female ascending Chlamydia infections and ovarian cancer in particular.




Comments