A collaborative study led by scientists from the Mechanobiology Institute (MBI) at the National University of Singapore (NUS) has revealed the mechanical forces that drive epithelial wound healing in the absence of cell supporting environment. This research was published in Nature Communications in January 2015.
Skin not only provides an essential protective barrier against foreign materials and pathogens, but it also helps the body retain various fluids and electrolytes. When this barrier is damaged, the consequences can be devastating. Ulcers, bleeding and bacterial infections may result and the chances of these occurring increases the longer wounds remain open.
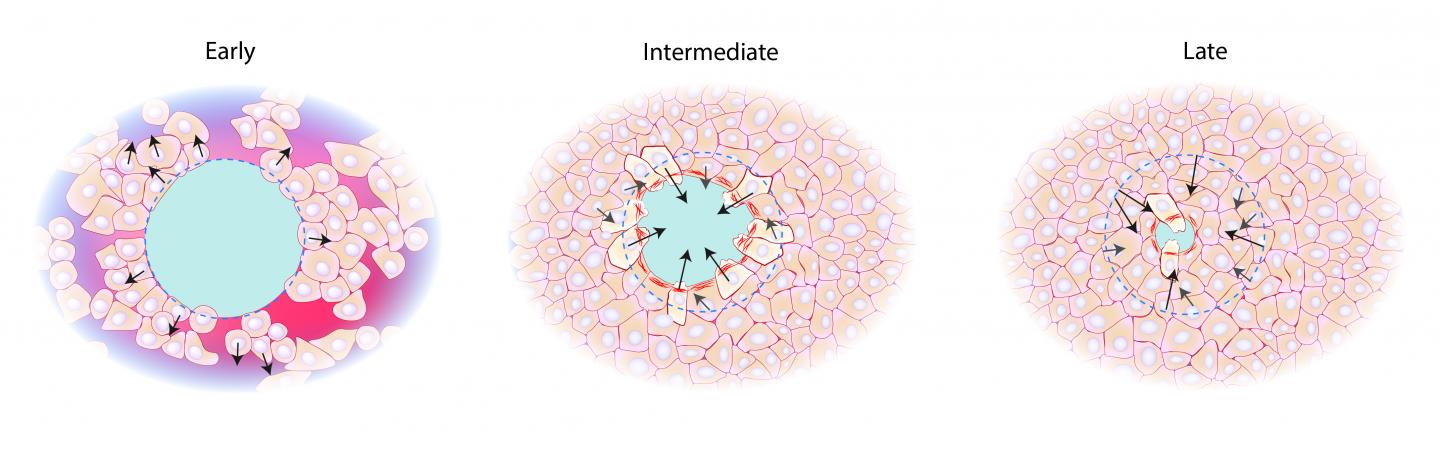
ces exerted by the cells surrounding the gap (dotted blue line) extend away at first, then direct inwards, towards the gap, during contraction of the 'purse-string' cable (red filaments). Credit: Mechanobiology Institute, National University of Singapore
Fortunately, epithelial cell sheets are self-repairing. The moment the integrity of the barrier is compromised, cellular mechanisms are initiated to close the gap. Cells begin crawling forward, and contractile cables are formed in the cells surrounding the wound to help pull the gap closed. For several years, scientists have been learning much about how cells coordinate these processes and repair wounds quickly. In most cases, the healthy skin cells responsible for carrying out wound repair rely on a supporting layer underneath them. This layer comprises sticky proteins, and is known as the extracellular matrix (ECM), which provides support for them to adhere to and crawl over.
However, in cases of chronic or severe wounds, the underlying layers could also be damaged. Surrounding cells could also be unable to replace the ECM proteins. Yet the repair of these gaps, known as non-adherent gaps, does occur, albeit at a slower rate and with an increased likelihood of infection or other complications. So the question remained; how do cells close gaps in protective epithelial barriers where the underlying layers are also damaged or the ECM eroded?
This question was the focus of a study led by MBI Principal Investigator (PI) Professor Chwee Teck Lim and Co-Principal Investigator Professor Benoit Ladoux, along with MBI PI Assistant Professor Yusuke Toyama. Their findings reveal that closure of non-adherent gaps is driven exclusively by 'purse-string contraction'. Using a combination of cell culture, microfabrication and force measurements, the scientists discovered that a cellular 'tug-of-war' at the gap edge drives the mechanical forces responsible for gap closure.
The cells at the edge of the non-adherent gap are still attached to the ECM. These cells then spread themselves out as far as possible towards the center of the gap. Measuring the direction of force revealed that these cells are actually pushing away from the gap. While this may sound counter-intuitive, it actually stabilizes the cells, in a similar manner to a cantilever bridge, where support at either end anchors the extension of the bridge into space until two sides eventually meet in the middle. Once the cells have spread as far as possible into the gap, the contractile 'purse-string' cable forms across the cells, encircling the gap.
The force exerted by these cells is reversed and the cells begin to pull each other towards the center of the gap, continually speeding up the contraction of the protein cable. As the cells move inwards to close the empty space, more contractile cables can reach out over the gap and connect to the other side. These cables can contract rapidly, leading to the formation of a suspended cell sheet over the gap, and complete closure of the wound.
The 'tug-of-war' mechanism identified in this study provides a vivid demonstration of how cells exert directional forces to enhance biological processes. This new knowledge of the mechanical properties of skin and internal epithelial cells may lead to advances in wound repair, especially in cases where the ECM is compromised. With chronic wounds, sores and ulcers being a common complication in several diseases, particularly those associated with aging, it is imperative that researchers better understand the mechanisms at play in their repair. This will undoubtedly lead to improved treatments in wound healing.




Comments