The loss of a gene in male mice results in the premature exhaustion of their fertility. Their fundamental new insights into the complex process of sperm generation may have direct applications to a similar loss of fertility in men.
What the team discovered is that the loss of the gene that makes the protein TAF4b causes a deficit in the number of progenitor cells at an embryonic stage of a male mouse's reproductive development. Lacking those important precursor cells means that the mice struggle to develop a robust stem cell infrastructure to sustain sperm production for the long term. The affected mice are fertile at first, but quickly deplete the limited sperm supply that they can generate.
"What's fascinating about these mice is they can reproduce," said Richard Freiman, senior author of the new study in the journal Stem Cells. "Mice can usually reproduce until they are two years old, but these mice can only reproduce until they are four months old."
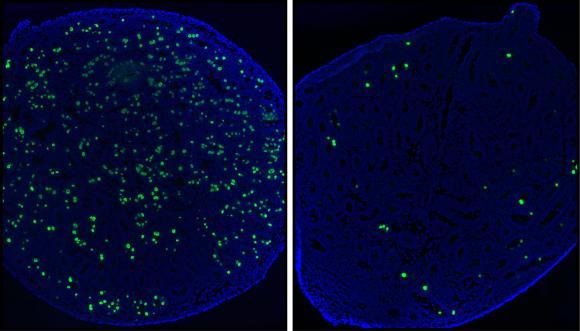
Mice lacking the gene for the protein TAF4b (right) produce fewer undifferentiated germ cells (green) than mice with the gene (left). Credit: Freiman lab/Brown University
TAF4b is a protein that affects how genes are regulated and transcribed, and its absence has profound impacts on the reproductive system. In previous work, Freiman's research group has shown that female mice without TAF4b are totally infertile and that their ovaries age prematurely. But in experiments with males, led by lab members Lindsay Lovasco and Eric Gustafson, the effect proved more subtle.
Sperm generation follows from a complex chain of events that the research shows begins before a male mouse is even born. In their experiments, the team compared the development of mice with and without the TAF4b gene. In mice with TAF4b, progenitor cells for sperm in the male embryo arise and proliferate normally, laying the groundwork in the testes for a robust pool of spermatogonial stem cells to develop. Those stem cells are the ones that produce a renewable supply of sperm. Without TAF4b, there were fewer progenitor cells and consequently fewer stem cells. They still produce sperm at first, but they can't renew production for the long haul. Ultimately the testes, which develop normally, become unproductive and atrophy.
What's not yet clear from the research is why the process fades out rather than just continuing, albeit at a very low level of productivity. One possibility is the low supply of spermatogonial stem cells drives the body to invest all its meager resources in immediate sperm production, leaving none of the stem cells in a more flexible state that can perpetually renew the supply. Another possibility is that regardless of supply, TAF4b is simply needed to see the renewal process through, for instance by maintaining some stem cells in their regenerative state.
Four Turkish brothers
Not only do humans have a gene for TAF4b, but a coincidental study last year in the Journal of Medical Genetics provides evidence that it also matters for sperm count. That research reported that four Turkish brothers who carried a mutation in the TAF4b gene had low sperm counts. Their mutation was in the same region of their gene as the one Freiman's team generated in the mice.
"The human implications are very exciting," he said. "It is possible that those men, as teenagers, were able to make functional sperm."
Certainly more research is needed, Freiman said, but if TAF4b mutation plays out in men the way it plays out in mice, his hope is that detecting the mutation in teenage boys could allow doctors to freeze their sperm so that when they are older and want to have children, they could draw on that banked supply.
"This is why fundamental knowledge is so important," Freiman said. "If we understand the process, we might be able to do something we couldn't do before."
The research also suggests the importance of development, even at the embryonic stage, to later life.
"Developmental processes that occur in embryogenesis really have a profound affect on the ability of adult organ systems to function properly," Freiman said. "This may be an example where a deficit during embryogenesis may preclude the ability of these mice to reproduce as adults."
In addition to Freiman, Lovasco and Gustafson, other authors are Kimberly Seymour of Brown and Dirk G. de Rooij of the Univerisity of Amsterdam.
The Ellison Medical Foundation and the National Institutes of Health (grant 1F32HD077986) supported the research.




Comments