Scientists have discovered that neurons use minor "DNA surgeries" to toggle their activity levels all day, every day, and since these activity levels are important in learning, memory and brain disorders, it could shed light on a range of important questions.
"We used to think that once a cell reaches full maturation, its DNA is totally stable, including the molecular tags attached to it to control its genes and maintain the cell's identity," says Hongjun Song, Ph.D., a professor of neurology and neuroscience in the Johns Hopkins University School of Medicine's Institute for Cell Engineering. "This research shows that some cells actually alter their DNA all the time, just to perform everyday functions."
This DNA alteration is called DNA demethylation. Methyl groups are regulatory tags that are permanently bound to cytosines, the C's in DNA's four-letter alphabet. Removing them is a multistep process that requires excising a tagged cytosine from the long string of paired "letters" that make up a chromosome and, ideally, replacing it with an untagged cytosine. Because the process involves making a cut into DNA, it leaves the DNA somewhat vulnerable to mutations, so most cells use the process sparingly, mostly for correcting errors. But recent studies had turned up evidence that mammals' brains exhibit highly dynamic DNA modification activity -- more than in any other area of the body -- and Song's group wanted to know why all this risky business was going on in such a vulnerable tissue as the brain.
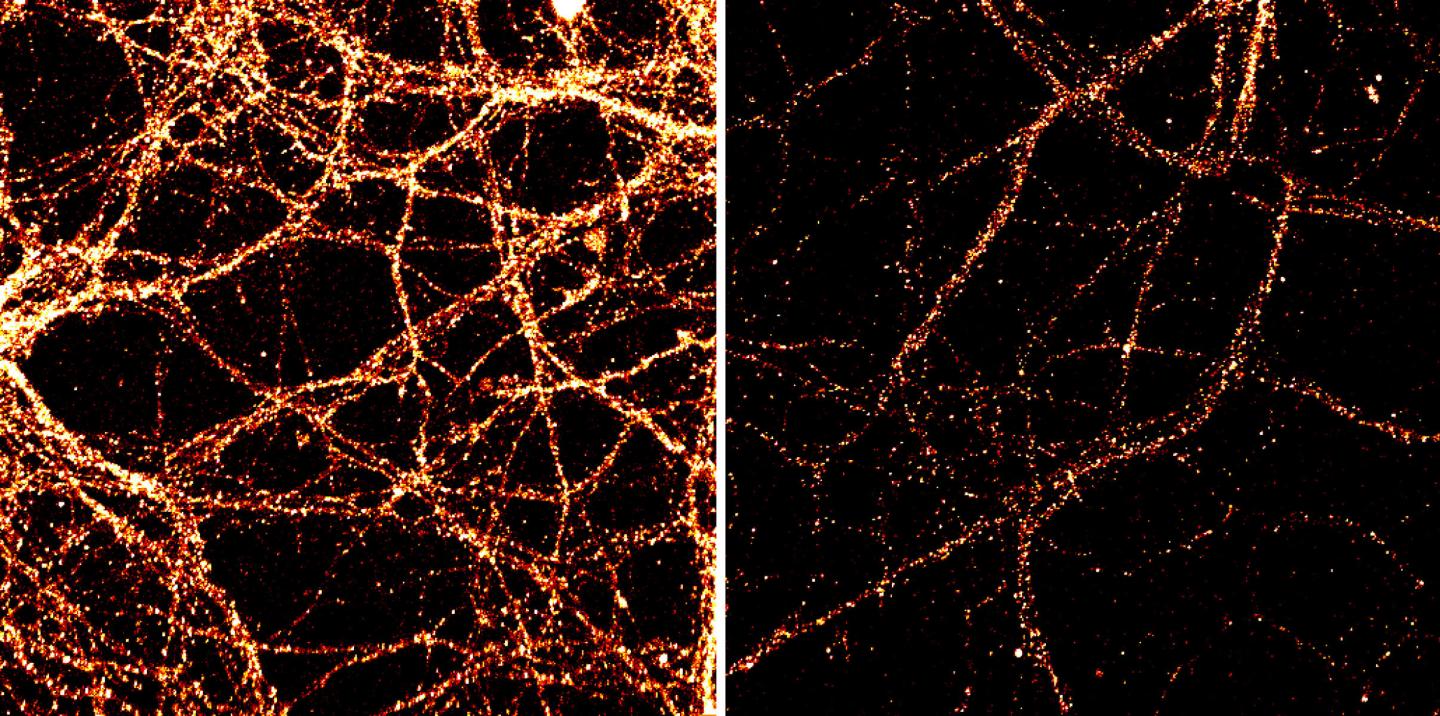
These are images of mouse neurons from the hippocampal region of the brain. Levels of the surface receptor GluR1, orange, are shown in unmodified neurons, left. Credit: Huimei Yu, Johns Hopkins Medicine
The main job of neurons is to communicate with other neurons through connections called synapses. At each synapse, an initiating neuron releases chemical messengers, which are intercepted by receptor proteins on the receiving neuron. Neurons can toggle the "volume" of this communication by adjusting the activity level of their genes to change the number of their messengers or receptors on the surface of the neuron. When Song's team added various drugs to neurons taken from mouse brains, their synaptic activity -- the volume of their communication -- went up and down accordingly. When it was up, so was the activity of the Tet3 gene, which kicks off DNA demethylation. When it was down, Tet3 was down too.
Then, they flipped the experiment around and manipulated the levels of Tet3 in the cells. Surprisingly, when Tet3 levels were up, synaptic activity was down; when Tet3 levels were down, synaptic activity was up. So do Tet3 levels depend on synaptic activity, or is it the other way around?
Another series of experiments showed them that one of the changes occurring in neurons in response to low levels of Tet3 was an increase in the protein GluR1 at their synapses. Since GluR1 is a receptor for chemical messengers, its abundance at synapses is one of the ways neurons can toggle their synaptic activity.
The scientists say they have discovered another mechanism used by neurons to maintain relatively consistent levels of synaptic activity so that neurons can remain responsive to the signaling around them. If synaptic activity increases, Tet3 activity and base excision of tagged cytosines increases. This causes the levels of GluR1 at synapses to decrease, in turn, which decreases their overall strength, bringing the synapses back to their previous activity level. The opposite can also happen, resulting in increasing synaptic activity in response to an initial decrease. So Tet3 levels respond to synaptic activity levels, and synaptic activity levels respond to Tet3 levels.
Song says: "If you shut off neural activity, the neurons 'turn up their volume' to try to get back to their usual level and vice versa. But they can't do it without Tet3."
Song adds that the ability to regulate synapse activity is the most fundamental property of neurons: "It's how our brains form circuits that contain information." Since this synaptic flexibility seems to require mildly risky DNA surgery to work, Song wonders if some brain disorders might arise from neurons losing their ability to "heal" properly after base excision. He thinks this study brings us one step closer to finding out.
Published in Nature Neuroscience. This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS047344, NS048271, NS062691), the National Institute of Mental Health (MH105128), the Simons Foundation (SFARI240011), the Brain and Behavior Research Foundation, the Maryland Stem Cell Research Foundation, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. Other authors of the report include Huimei Yu, Yijin Su, Jaehoon Shin, Chun Zhong, Junjie Guo, Yi-lan Wen and Guo-li Ming of the Johns Hopkins University School of Medicine; and Fuying Gao, Daniel Geschwind and Giovanni Coppola of the University of California, Los Angeles.




Comments